Is Sulfur Trioxide (SO3) Polar or NonPolar? Lewis Structure (The

Is SO3 Polar or Nonpolar? Techiescientist
Sulfur trioxide is a compound with the chemical formula SO3. This compound is of great importance and studied widely as it reacts with the water present in the air to produce sulfuric acid. When this sulfuric acid, in the gaseous state, mixes with the rain and falls on the Earth, it is called acid rain.
Is Sulfur Trioxide (SO3) Polar or NonPolar? Lewis Structure (The
DO NOT FORGET TO SUBSCRIBE!LinkedIn: https://www.linkedin.com/in/kevan-j-e.Snapchat: https://www.snapchat.com/add/kravonoInstagram: https://www.instagram.c.

dipole moment SO2 and SO3 Polar NonPolar YouTube
Henry Agnew (UC Davis) 5.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken if energy is added to a molecule.

So3 Polar or Nonpolar XavierhasTran
Sulfur Trioxide comprises one Sulfur and three Oxygen atoms. To determine its polarity, we will first look at its Lewis Structure to see the atoms' arrangeme.

Is SO3 Polar or Nonpolar? Polarity of SO3
The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Calculate the electronegativity difference (ΔEN) and average ( EN) of the two electronegativities, and use the table below to determine the bond type and polarity. Calculate the molecular polarity (polar, non-polar) of a chemical bond based.

Is SO3 Polar or Nonpolar? Polarity of SO3
Answer and Explanation: 1. Become a Study.com member to unlock this answer! Create your account. View this answer. Sulfur trioxide is an inorganic molecule composed of four atoms. Three of those atoms are oxygen, and one is sulfur. The sulfur atom is the central. See full answer below.

Is SO3 Polar or Nonpolar? Techiescientist
If there is only one bond in the molecule, the bond polarity determines the molecular polarity.Any diatomic molecule in which the two atoms are the same element must be a nonpolar molecule. A diatomic molecule that consists of a polar covalent bond, such as HF, is a polar molecule where one end of the molecule is slightly positive, while the other end is slightly negative.
MakeTheBrainHappy Is SO3 Polar or Nonpolar?
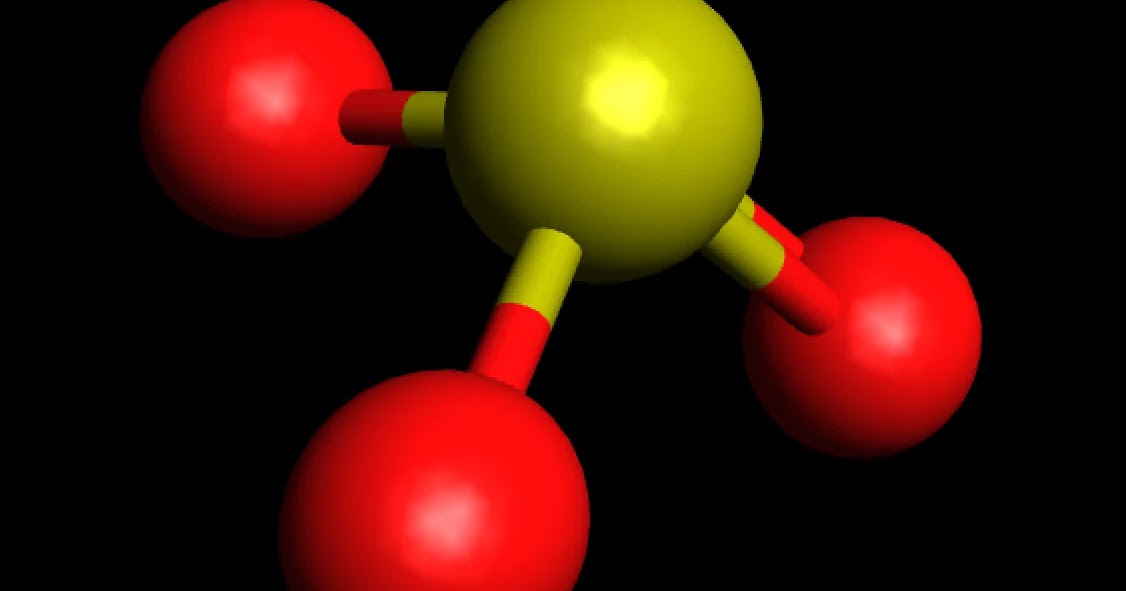
The molecule SO 3 is trigonal planar. As predicted by VSEPR theory, its structure belongs to the D 3h point group. The sulfur atom has an oxidation state of +6 and may be assigned a formal charge value as low as 0 (if all three sulfur-oxygen bonds are assumed to be double bonds) or as high as +2 (if the Octet Rule is assumed). [7]
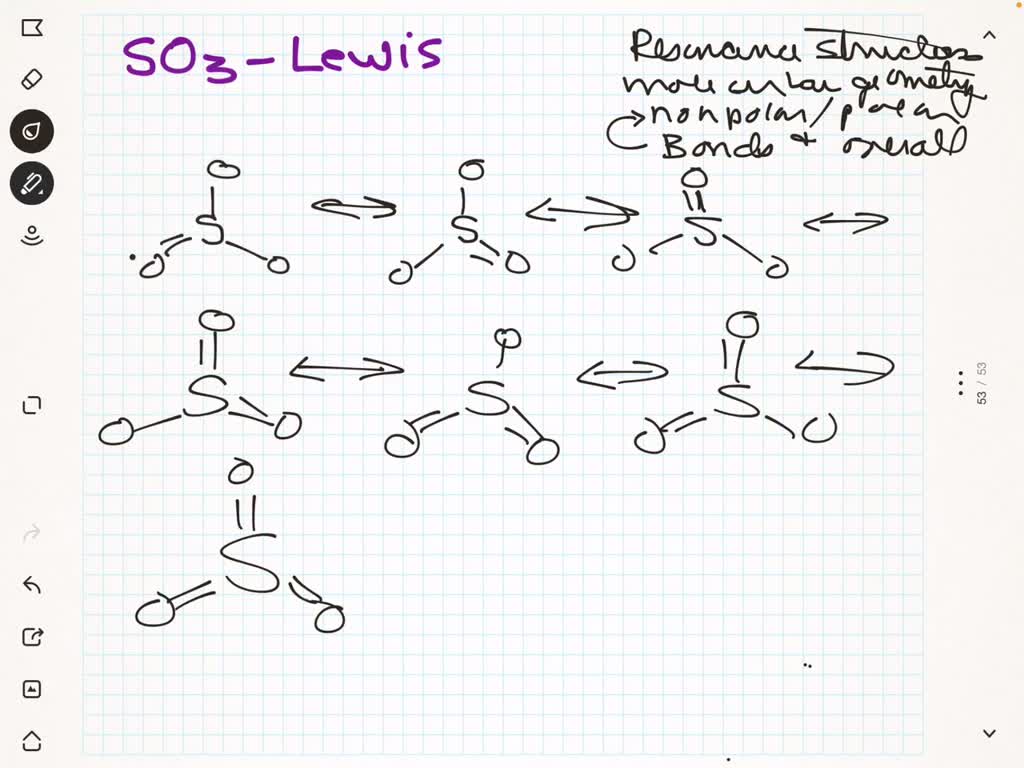
SOLVED a. Give the Lewis electron dot structure for SO3. If there are
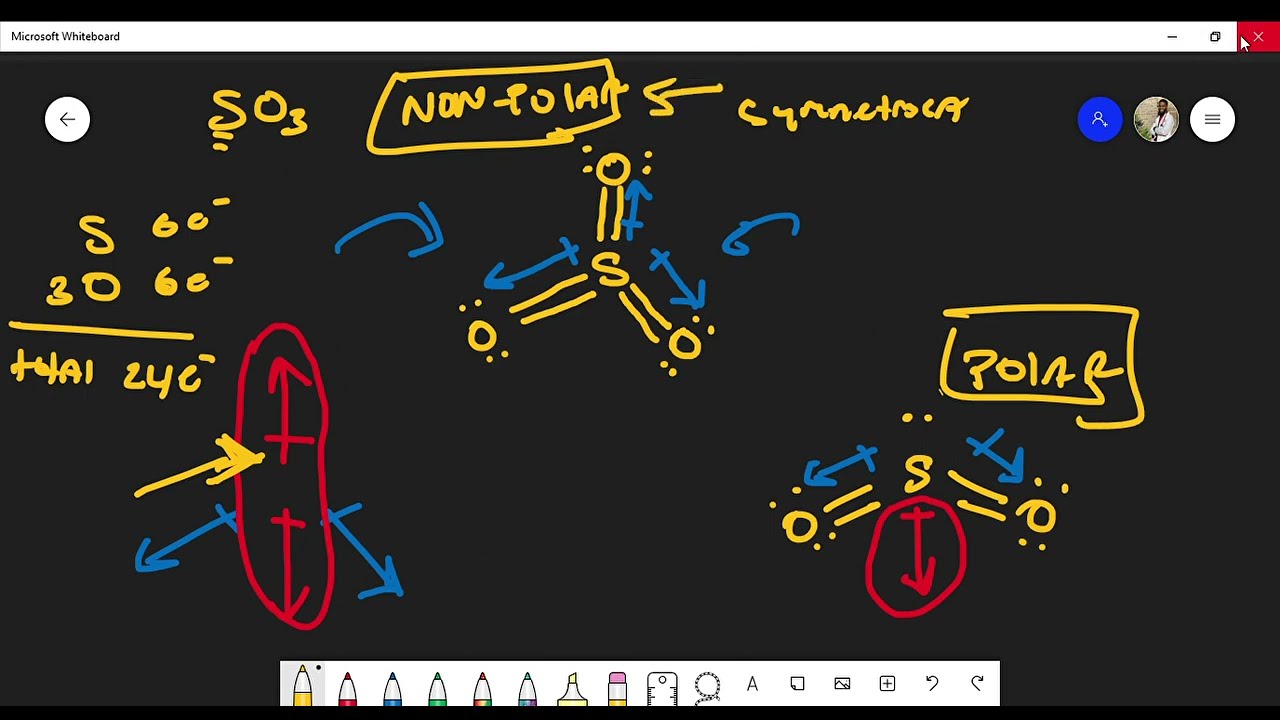
SO3 is a NONPOLAR molecule because all the three bonds (S=O bonds) are identical and SO3 has symmetrical geometry which cancels out the bond polarity. Let me explain this in detail with the help of SO3 lewis structure and its 3D geometry. Why is SO3 a Nonpolar molecule? (Explained in 3 Steps)

Best Overview Is SO3 Polar or Nonpolar? Science Education and Tutorials
SO3 2- (Sulfite ion) is a POLAR ion. But why? And how can you say that SO3 2- ion is a polar ion? Want to know the reason? Let's dive into it! SO3 2- is a POLAR ion because the Oxygen (O) atom is more electronegative and it also has lone pair, which results in an asymmetric shape of the ion.

Is SO3 Polar or NonPolar?
As this difference lies between 0.4 and 1.6, the bonds formed in the SO3 molecule are polar covalent bonds. However, the net charge on the sulfur trioxide molecule is zero owing to its geometry, due to which the molecule in itself is non-polar.

Trigonal Planar Hybridization
SO3 which is also spelled as Sulphur Trioxide sometimes, is a trigonal planar molecule that is non-flammable. In this article, I will provide you some information regarding SO3 molecular geometry with the explanations of Lewis structure, polarity, and hybridization. Contents Sulfur Trioxide Molecular Geometry Lewis Structure of SO3 Polarity of SO3

Is SO3 Polar or Nonpolar? Polarity of SO3
This video explains the dipole moment of sulphur dioxide and sulphur trioxide. From the dipole moment value we also can predict the molecule is polar or non.

So3 Polar or Nonpolar NicoabbGuerrero
The chemical substance sulphur trioxide (also known as nisso sulfan) has the formula SO 3 (alternative spelling: sulphur trioxide). "Unquestionably the most significant commercially", according to Sulphur Oxide. It is produced in enormous quantities as a precursor to sulphuric acid.

Is SO3 Polar or Nonpolar? Polarity of SO3
SO3 is an oxide of sulfur and is the primary pollutant present in the atmosphere. When mixed with water vapors in the air, Sulfur trioxide leads to the formation of sulfuric acid, thus contributing to acid rain formation. A lot of students ask about the polarity of the so3 molecule.

so3 2 polar or nonpolar
Explanation 1: The sulfur trioxide (SO3) is a nonpolar molecule because, in SO3, electrons' sharing is equal, and the lewis structure of SO3 appears to be an asymmetrical molecule. Explanation 2: The sulfur trioxide (SO3) is a nonpolar molecule because the shape of the SO3 is trigonal planar.