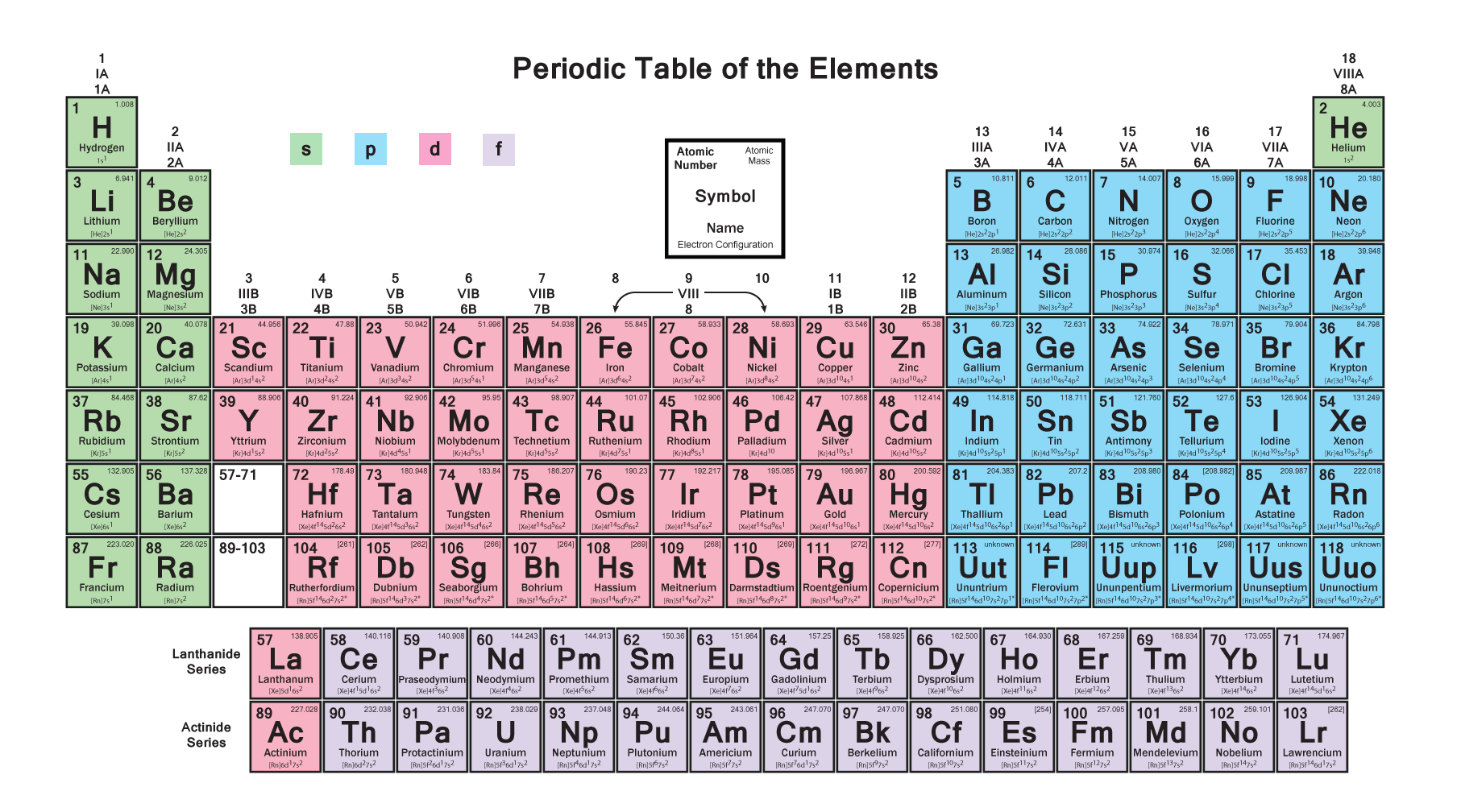
Electron configuration example for phosphorus Science, Chemistry
These Daily Uses of pblock Elements will Surprise You askIITians
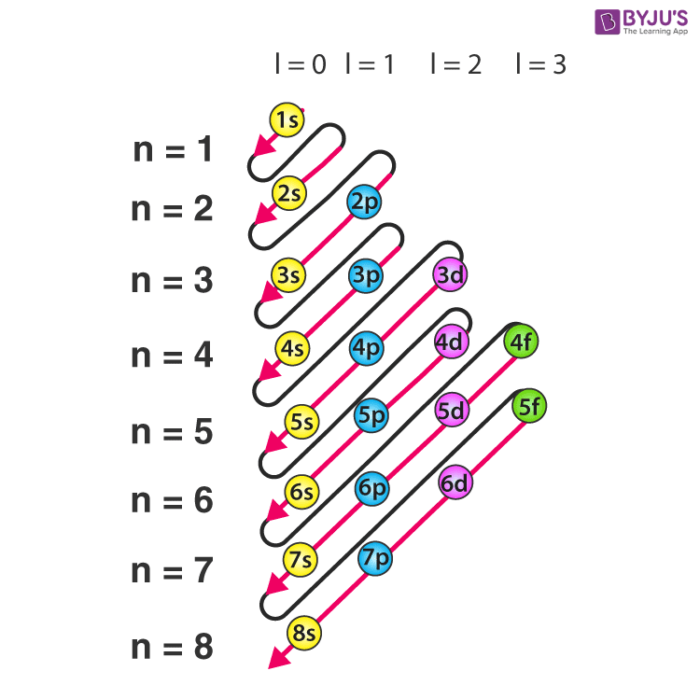
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.

Electronic Configuration Of Elements Trick (s,p,d,f) Pattern Class11
This electron configuration is written as 1 s2 2 s1. The next element is beryllium, with Z = 4 and four electrons. We fill both the 1 s and 2 s orbitals to achieve a 1 s2 2 s2 electron configuration: When we reach boron, with Z = 5 and five electrons, we must place the fifth electron in one of the 2 p orbitals.
Electron configuration example for phosphorus Science, Chemistry
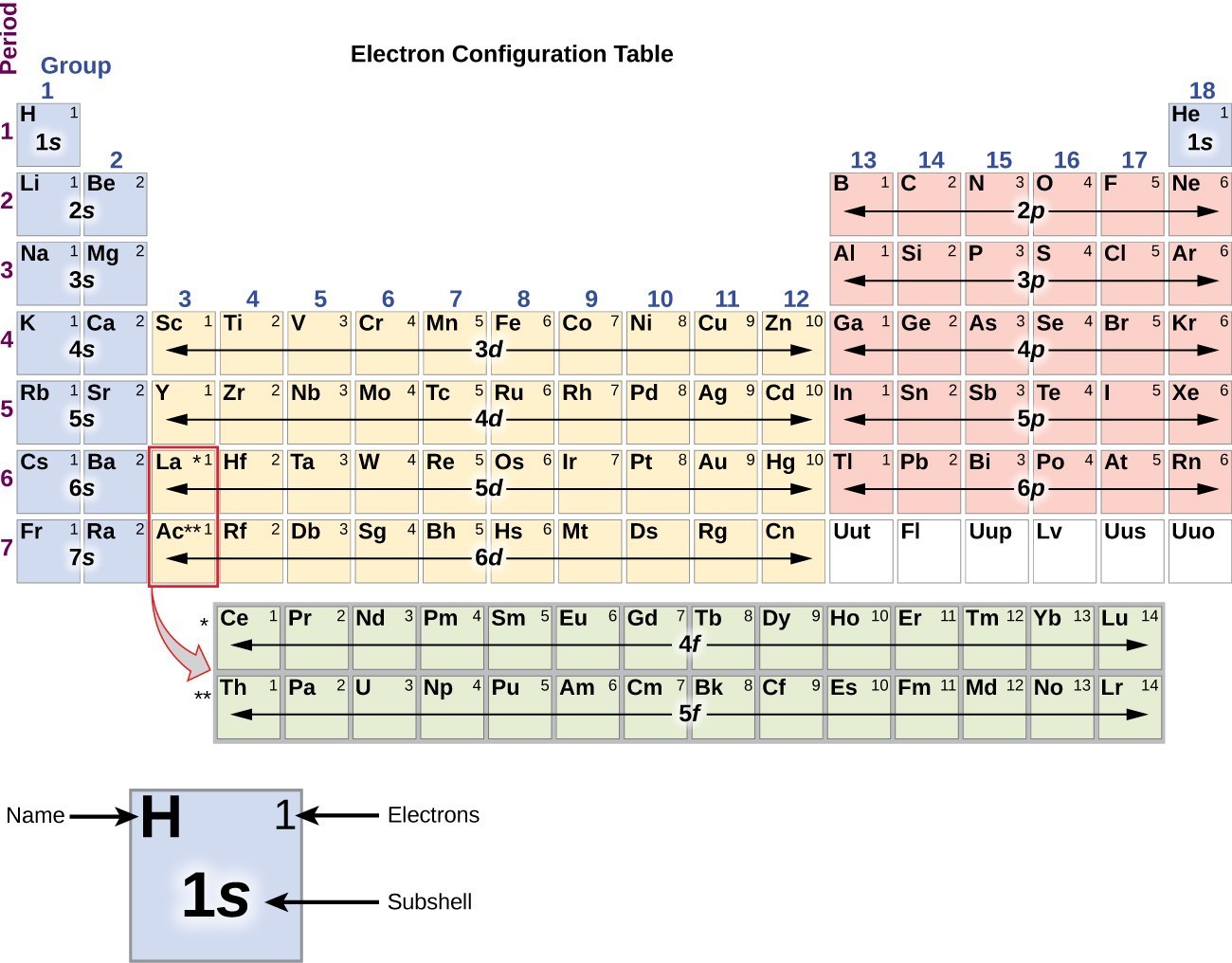
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1#

Electronic Configurations Intro Chemistry LibreTexts
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). You will get the detailed information about the periodic table which will convert a newbie into pro. 3). You will also get the HD images of the Periodic table (for FREE).
Structura electronica a Atomilor (Configurații de Electroni) Chimie
The arrangement of electrons in phosphorus in specific rules in different orbits and orbitals is called the electron configuration of phosphorus. The electron configuration of phosphorus is [ Ne] 3s 2 3p 3 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways.

6.9 Elektronové konfigurace a periodická tabulka Chemistry
In this video we will write the electron configuration for Phosphours (P) and P 3- (the Phosphide ion). We'll also look at why Phosphorous forms a 3- ion and.
Electronic configuration detailed explanation, orbital filling
AboutTranscript. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan.
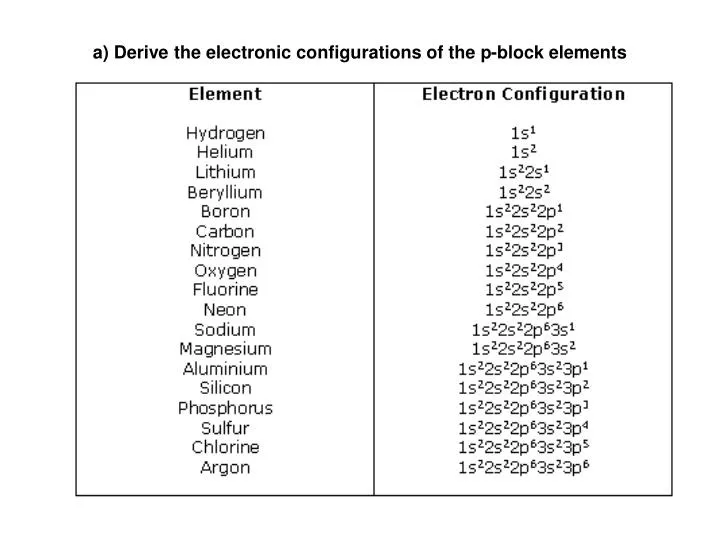
PPT a) Derive the electronic configurations of the pblock elements
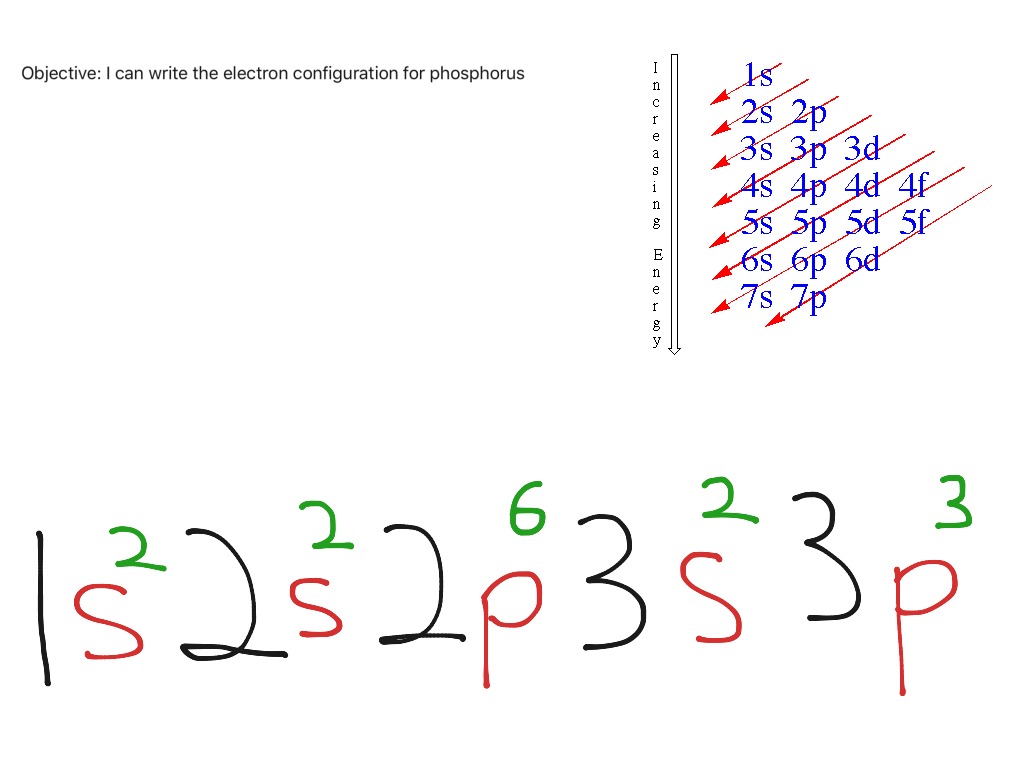
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period table or an electron configuration chart. How to Write the Electron Configuration for Phosphorus (P)
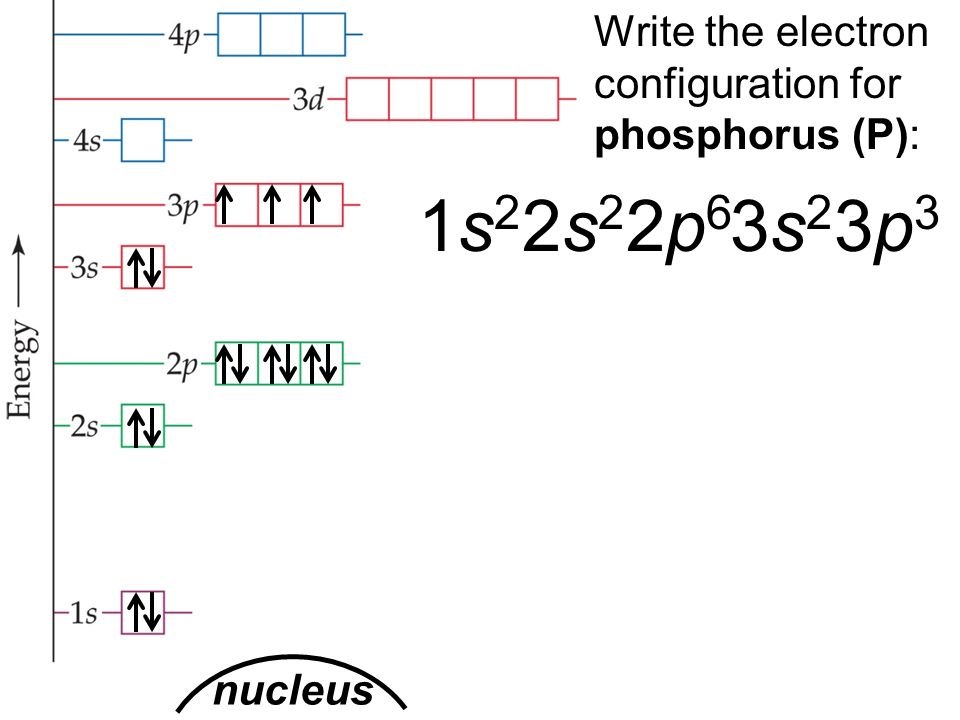
Phosphorus Electron Configuration (P) with Orbital Diagram
The valence electron configuration for phosphorus is s^2 p^3. Phosphorus has an electron configuration of 1s^2 2s^2 2p^6, 3s^2 3p^3. Phosphorus is found in group 15, the other non-metals on the periodic table. Phosphorus is in the 3rd energy level, (3rd row) and 3rd column of the 'p' block 3p^3. The valence electrons are always found in the 's' and 'p' orbitals of the highest energy level of.
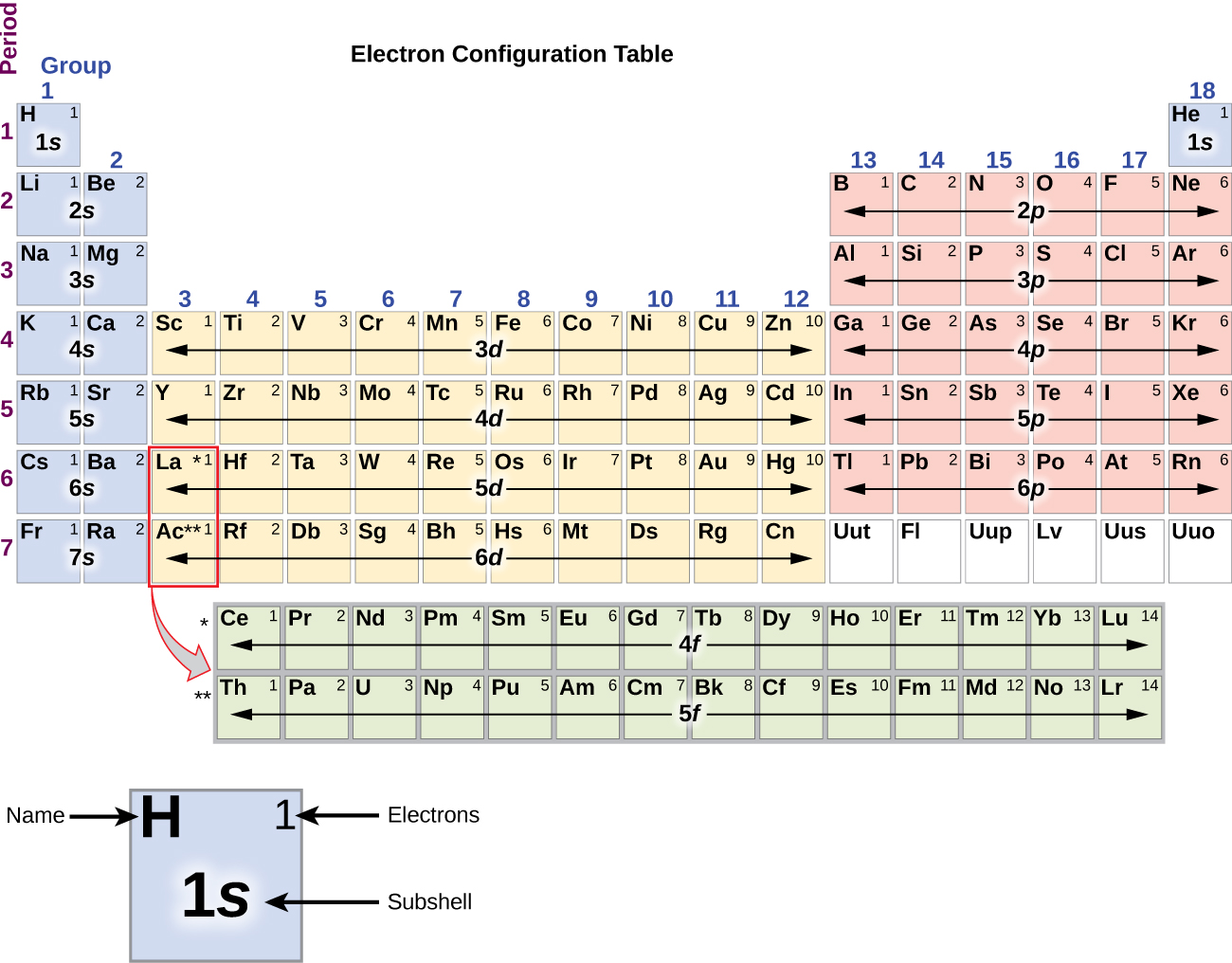
Electron configuration periodic table qustbh
0:00 / 1:45 A step-by-step description of how to write the electron configuration for Phosphorus (P). In order to write the P electron configuration we first need to kn.
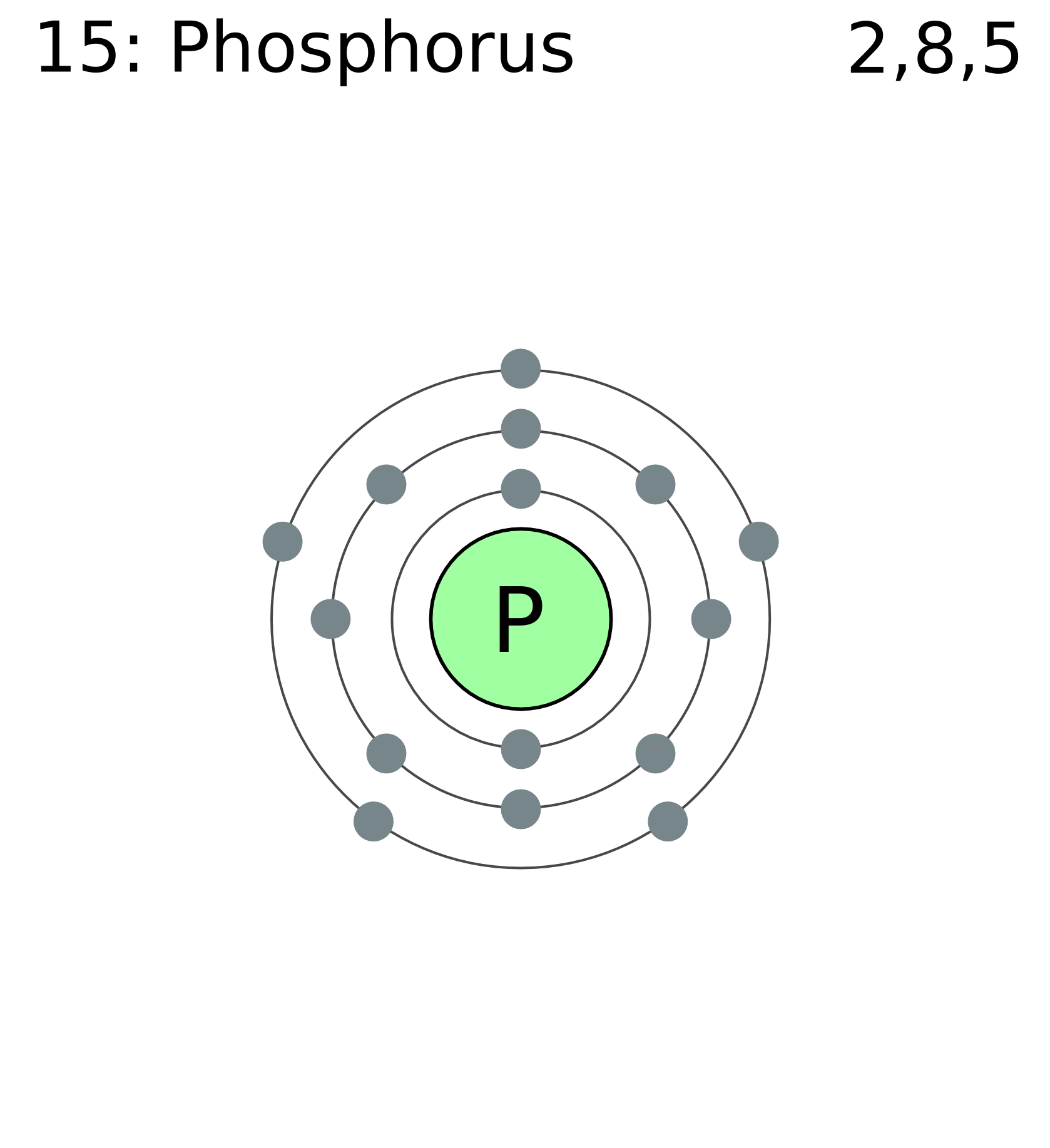
Phosphorus Electron Configuration (P) with Orbital Diagram
Electronic configurations describe each electron as moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by
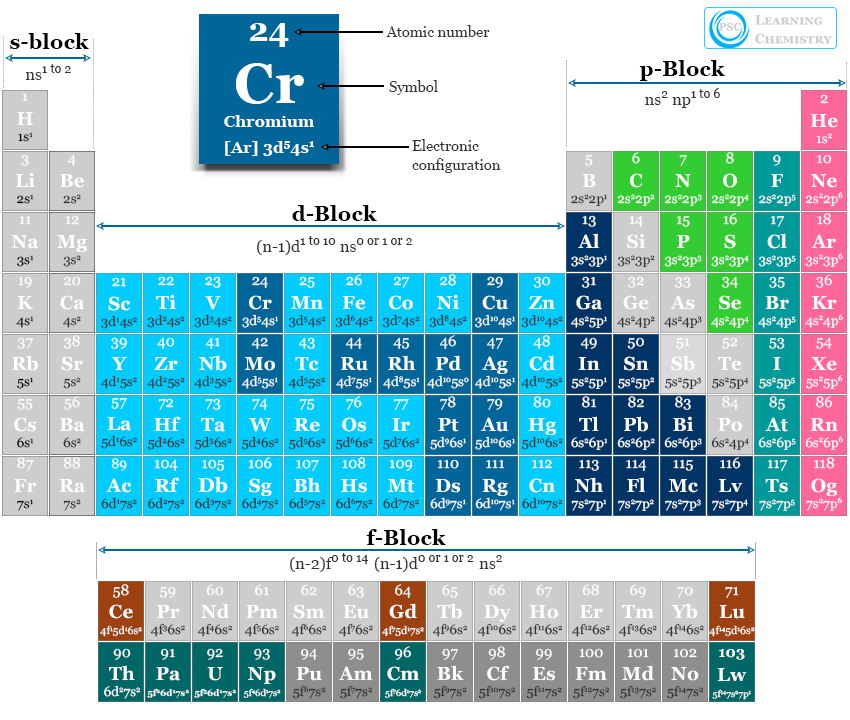
Electron Configuration Periodic Table Elements Chemistry
The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). For instance, the ground state electronic configuration of calcium (Z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.

Write the general outer electronic configuration of `s`,`p`,`d` and
Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. As we know, the positively-charged protons in the nucleus of an atom tend to attract negatively-charged electrons.
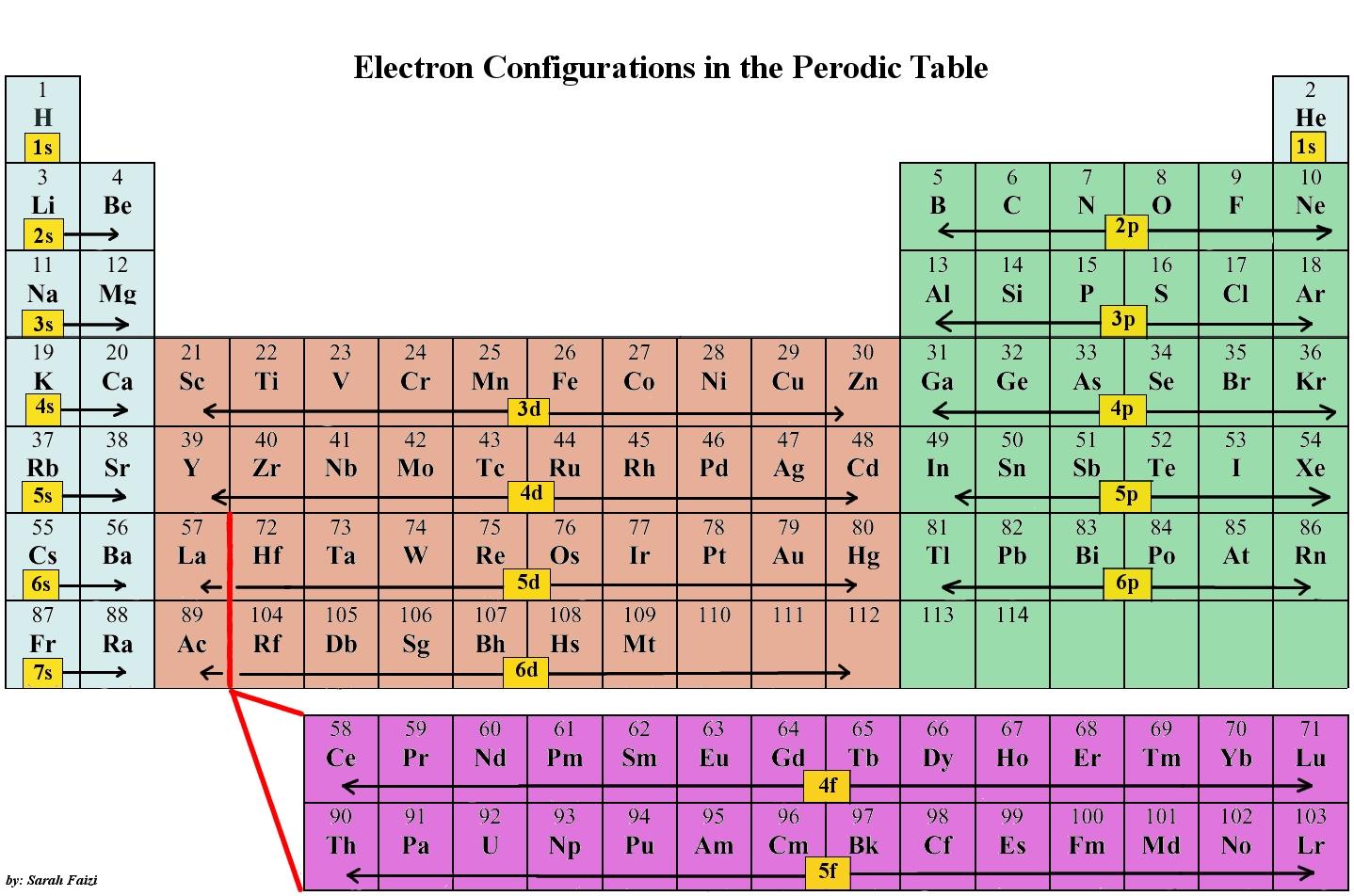
Electron Structure ALevel Chemistry Revision Notes
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms= + 1 2 1 2 ).
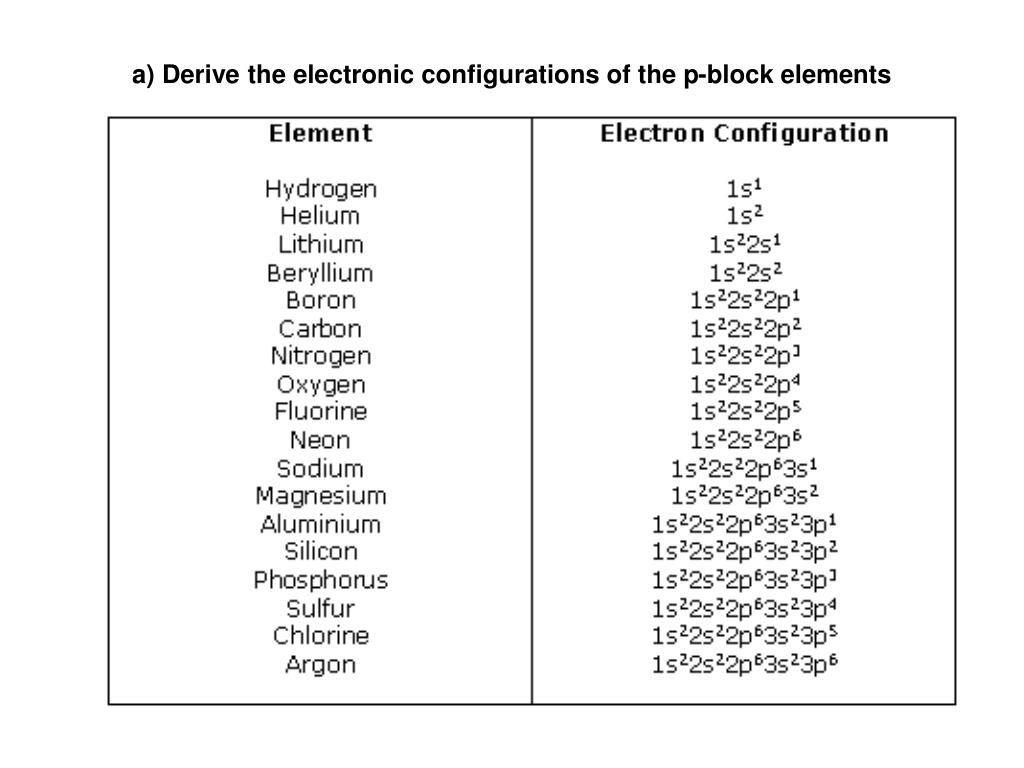
PPT a) Derive the electronic configurations of the pblock elements
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the building-up process for the lanthanoids.

Electronic Configuration of a Atom Atomic Structure Electronic
This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the concise form is [Ne] 3s 2 3p 3.