NO3 Lewis Structure, Molecular Geometry, and Hybridization

Lewis Structure NO3 plus dipoles, shape, angles, resonance and formal
NO3- (nitrate ion) lewis structure has a Nitrogen atom (N) at the center which is surrounded by three Oxygen atoms (O). There is 1 double bond and 2 single bonds between the Nitrogen atom (N) and each Oxygen atom (O).. In the above lewis dot structure of NO3- ion, you can also represent each bonding electron pair (:) as a single bond (|). By.
14 Intriguing Facts About Lewis Dot Structure
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion.
NO3 Lewis Structure How to Draw the Lewis Structure for NO3 YouTube
This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Top No3 Lewis Structure Molecular Geometry Bond Angles Background GM
Lewis structure of NO3- ion (nitrate ion) contains one double bond and two single bonds between the Nitrogen (N) atom and Oxygen (O) atoms. The Nitrogen atom (N) is at the center and it is surrounded by 3 Oxygen atoms (O). Let's draw and understand this lewis dot structure step by step.

Lewis structure of NO3 (Nitrate ion)Draw the Lewis dot structure of
A simple procedure for writing Lewis Dot Structures is shown in this video.Several worked examples relevant to this procedure are given.http://chem-net.blogs.

SOLVED Draw the Lewis Dot Structure for nitrate, NO31
Lewis Dot Structure of NO3- (Nitrate Ion) kentchemistry.com 25.1K subscribers Subscribe Subscribed 294K views 12 years ago Every Video I quickly take you through how to draw the Lewis.

someone plz send the lewis dot structure of NO3 Chemistry
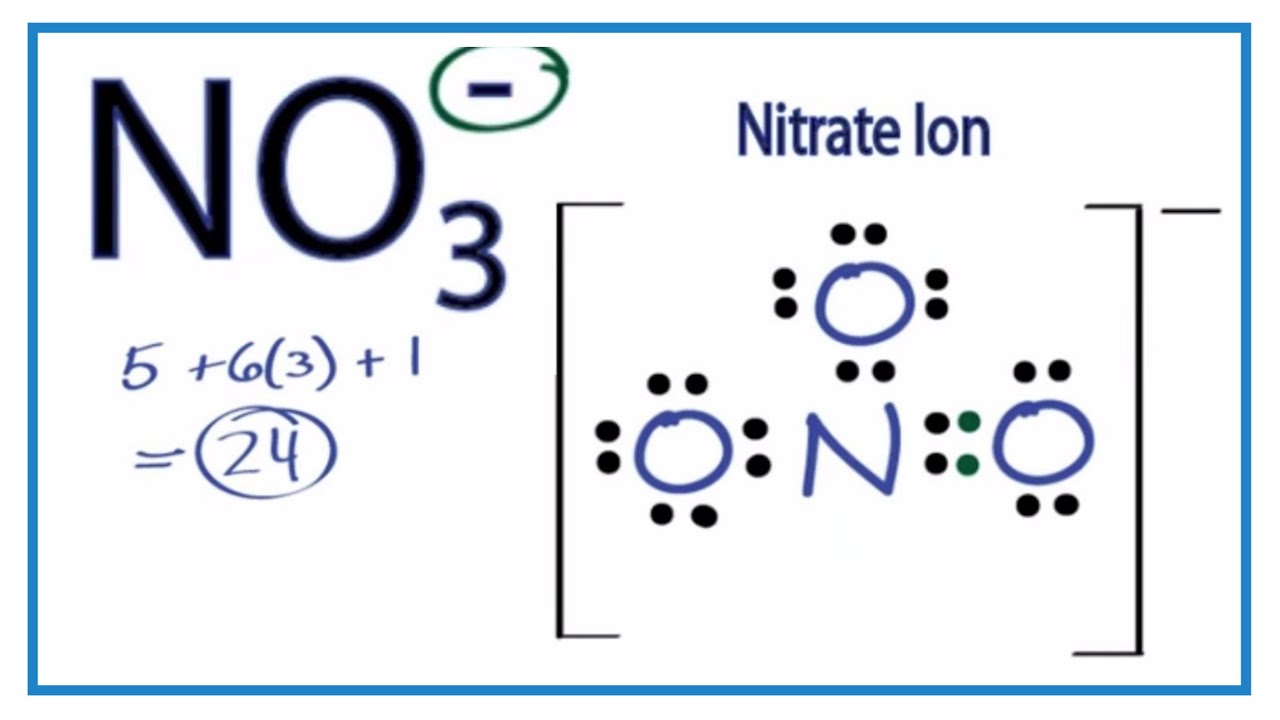
Following steps are required to draw NO 3- lewis structure and they are explained in detail in this tutorial. Find total number of electrons of the valance shells of nitrogen and oxygen atoms and including charge of the anion Total electrons pairs in valence shells Center atom selection from nitrogen and oxygen atom Put lone pairs on atoms

NO3 Lewis Structure, Molecular Geometry, and Hybridization
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

NO3 Molecular Geometry / Shape and Bond Angles YouTube
Construction of NO3 Lewis Dot Structure 1. In the ion NO3, there is 1 atom of nitrogen and 3 atoms of oxygen. It also has one negative charge. 2. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the periodic table. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell.

How To Draw The Lewis Dot Structure For No3 Nitrate Ion
The NO3- Lewis structure represents the nitrate ion, which consists of one nitrogen atom and three oxygen atoms. The central nitrogen atom forms one double bond and two single bonds with the three surrounding oxygen atoms.
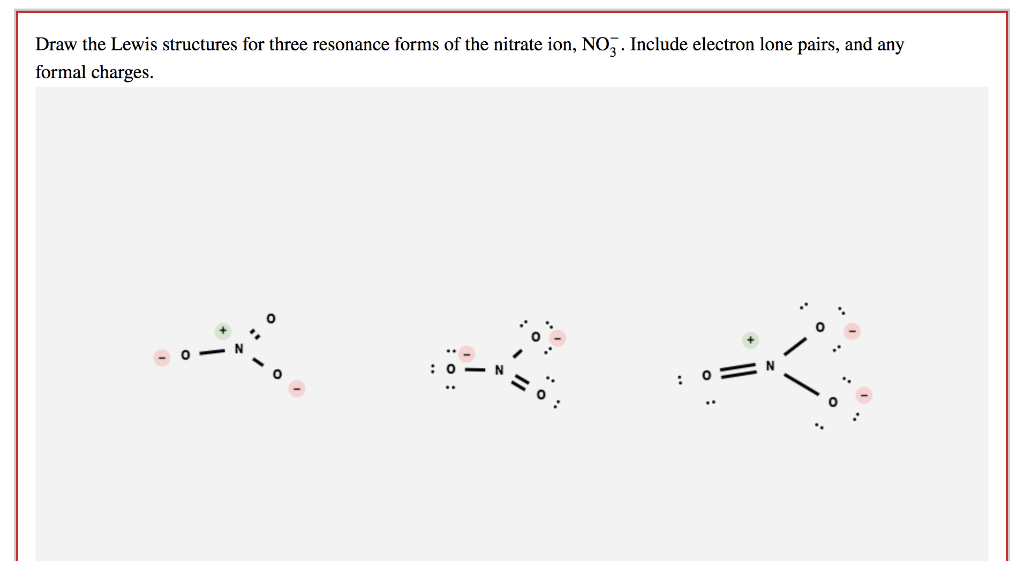
Draw The Lewis Structures For Three Resonance Forms Chegg Com My XXX
How to Draw the Lewis Dot Structure for NO3 - (Nitrate ion) - YouTube A step-by-step explanation of how to draw the NO3- Lewis Dot Structure (the Nitrate ion).For the NO3 - structure.

Drawing Lewis Dot Structures For Covalent Compounds Youtube
There are 24 valence electrons available for the Lewis structure for NO 3-. Video: Drawing the Lewis Structure for NO3- n It is helpful if you: Try to draw the NO 3- Lewis structure before watching the video. Watch the video and see if you missed any steps or information.

How to Draw the Lewis Dot Structure for Sr(NO3)2 Strontium nitrate
A step-by-step explanation of how to draw the NO3- Lewis Dot Structure (Nitrate ion).For the NO3- structure use the periodic table to find the total number o.

what is resonance?resonating structure of NO3 ion Brainly.in
Transcript: This is the NO3- Lewis structure: the nitrate ion. Nitrogen has 5 valence electrons. Oxygen has 6, we have 3 Oxygens, and we need to add 1 for this valence electron up here. That gives us a total of 5 plus 18 plus 1: 24 valence electrons. Nitrogen is the least electronegative; we'll put that in the center, and we'll put the Oxygens.

How To Draw The Lewis Structure of SO4 2 (Sulfate Ion) YouTube
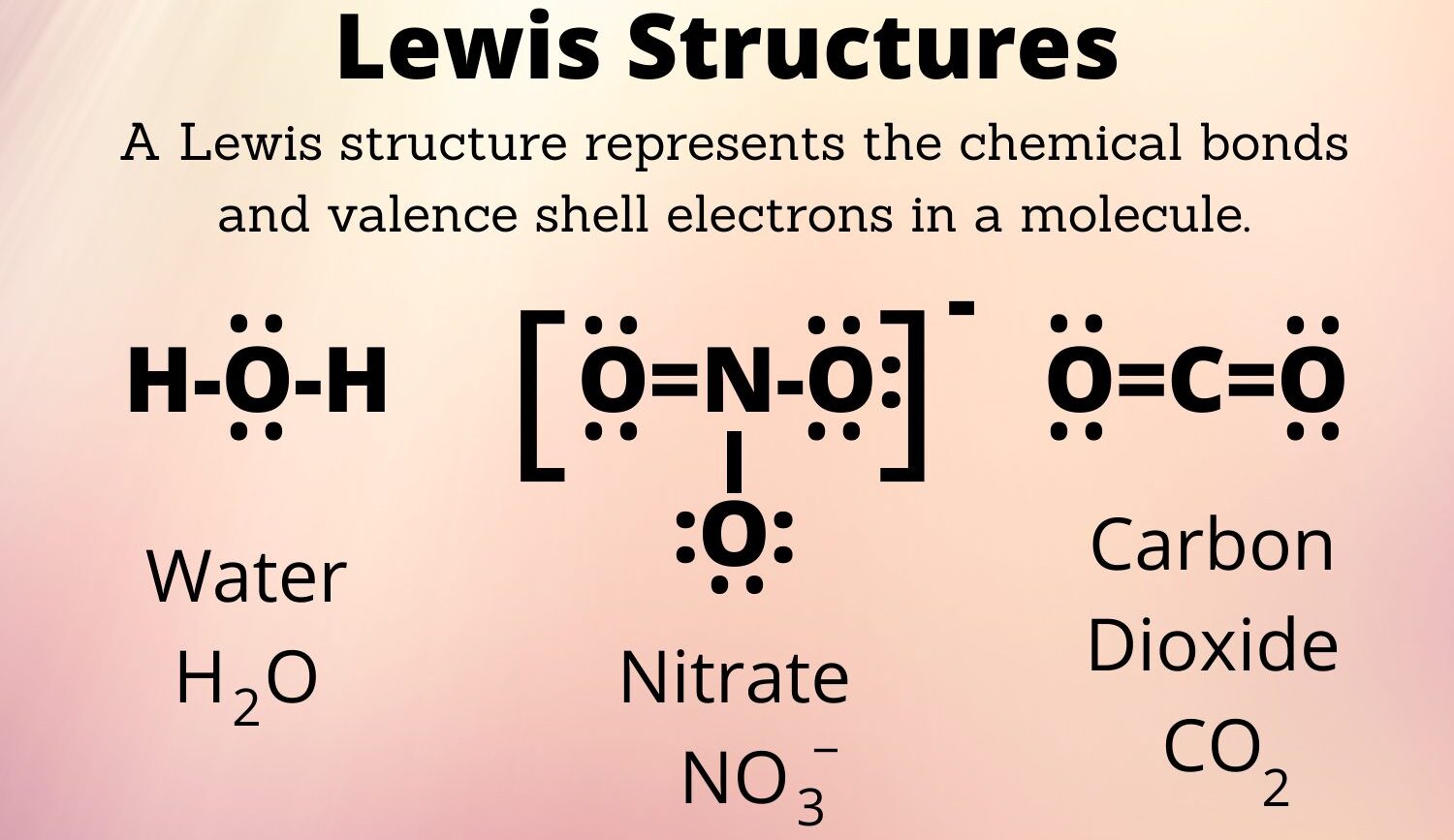
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
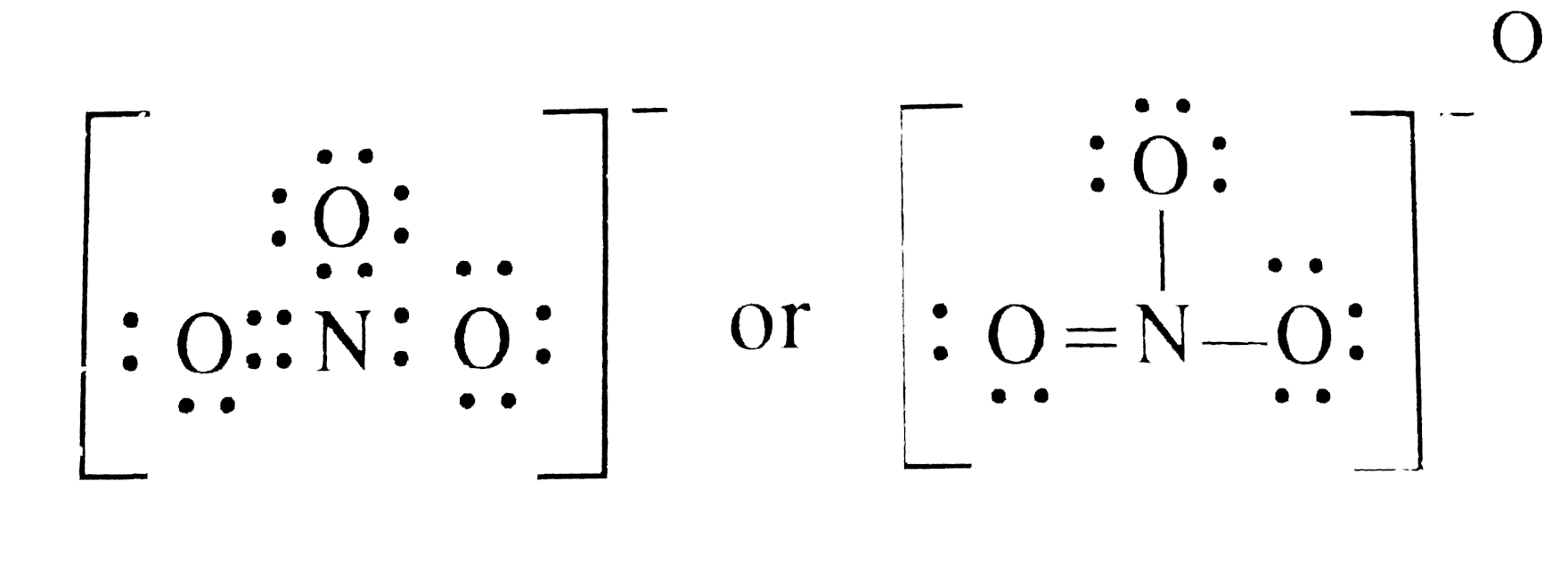
How To Draw The Lewis Dot Structure For No3 Nitrate Ion
Lewis structure of a water molecule. Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as.