O3 Lewis Structure Step By Step Drawing What's Insight

confusion over resonance major contributor and reactivity r/Mcat
Lewis Structure To be very precise, Lewis Structure is the name given to the structural representation of a molecule. It is the diagrammatic layout for understanding the nitty-gritty of chemical bonding. A very essential concept of molecular chemistry, the following steps dictate how you can successfully draw Lewis Structure: Step 1
O3 ozone molecule T & L EQUIPMENT SALES CO., INC. Commercial
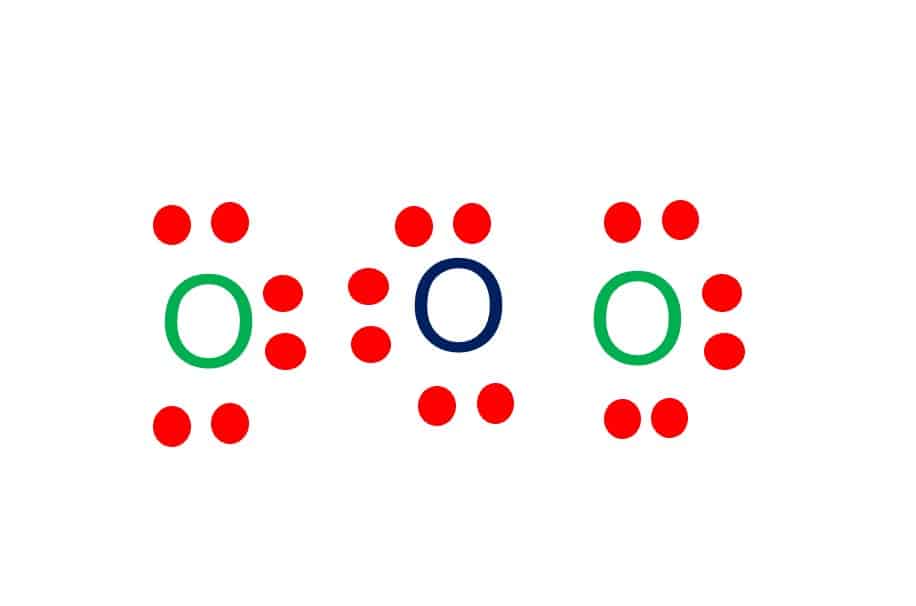
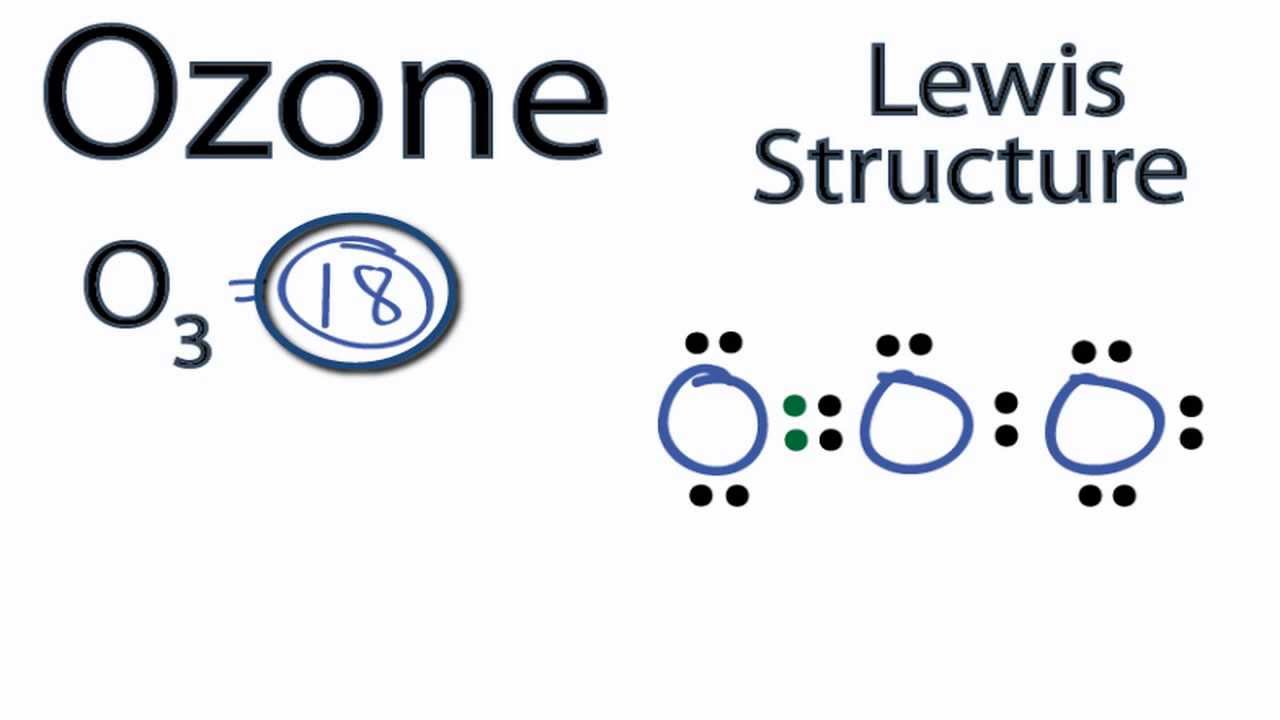
Dots around the central atom represent these electrons. Contents O3 Valence electrons O3 Lewis Structure Resonance structures of O3 O3 Hybridization O3 Molecular Geometry O3 Bond Angles O3 Shape O3 Polar or Nonpolar O3 Valence electrons In Ozone or O3, there are six valence electrons for each molecule of Oxygen.
O3 Lewis Structure Step By Step Drawing What's Insight
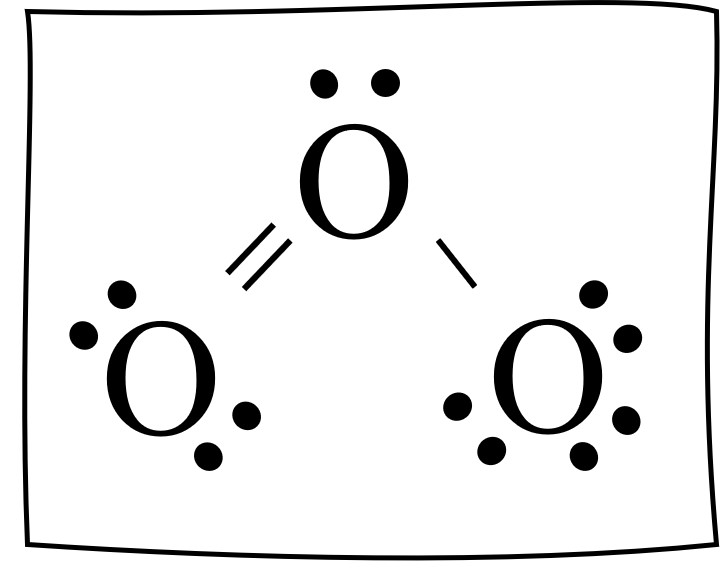
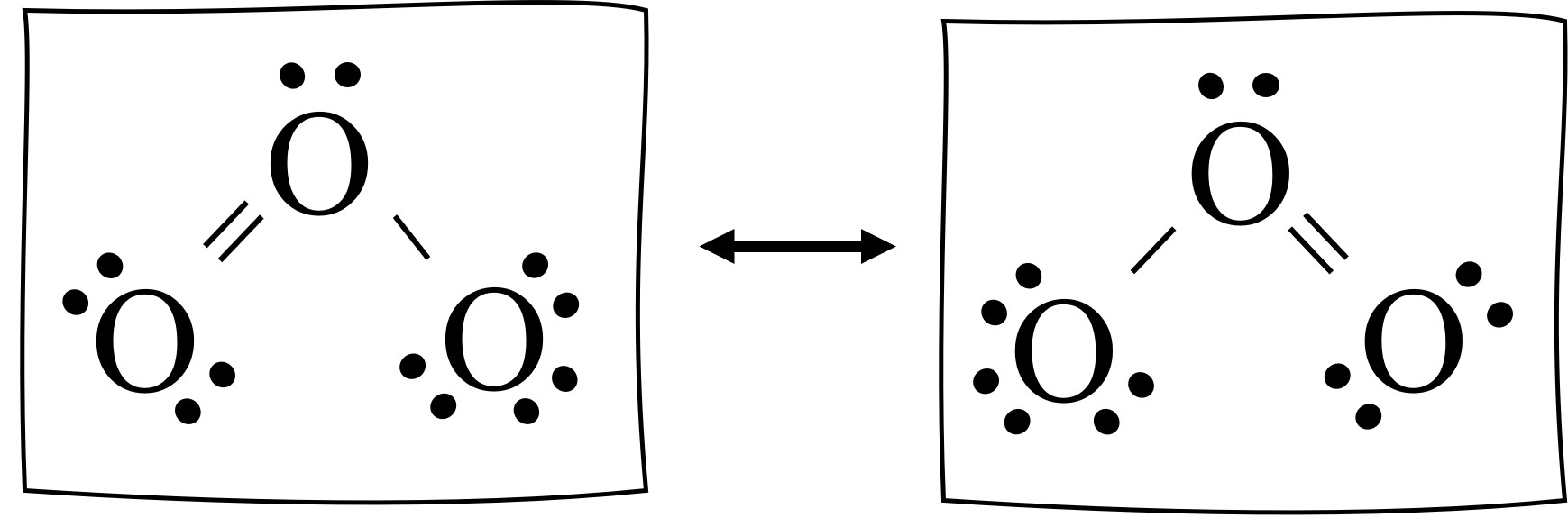
O3 lewis structure has three Oxygen atoms (O). There is 1 double bond and 1 single bond between the Oxygen atoms (O). There is 1 lone pair on the central. Hence the above lewis structure of O3 is stable. In the above lewis dot structure of O3, you can also represent each bonding electron pair (:) as a single bond (|). By doing so, you will.
Resonance Structures Easy Hard Science
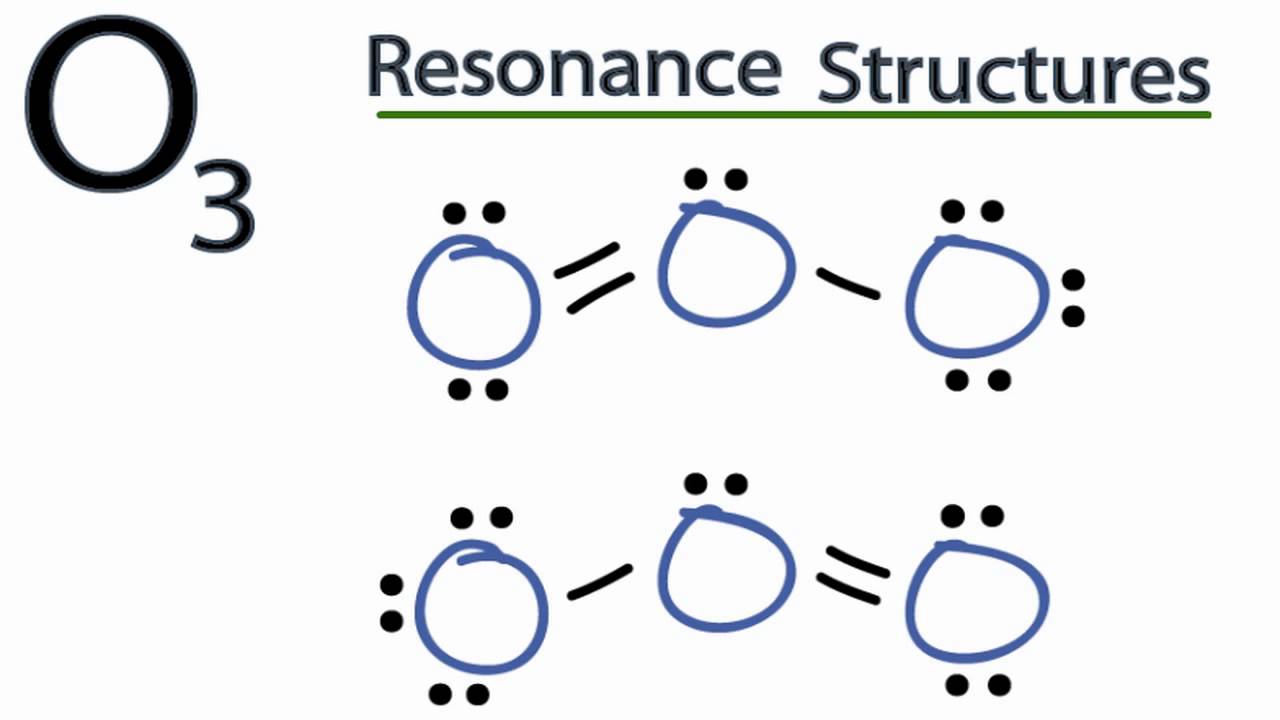
Drawing the Lewis Structure for O 3. Viewing Notes: For the Lewis Structure for O 3 it is possible to draw it two different says (slightly different, but still important). These are called resonance structures. O 3 (Ozone) is important in the upper atomsphere since it blocks UV light that can be harmful to humans (for example: causing skin cancer).; Be sure that you don't use more than the 18.
The 25+ Reasons for Ozone Structure This causes ozone to have
California's 37th congressional district is a congressional district in the U.S. state of California based in Los Angeles County.It includes many neighborhoods west and southwest of Downtown Los Angeles.. The district includes Culver City; Inglewood; the City of Los Angeles neighborhoods of Mid City, Century City, Beverlywood, View Park-Windsor Hills, Pico-Robertson, Exposition Park.
Resonance Structures Easy Hard Science
In the Lewis structure of O3, we can see that each oxygen atom has six electrons around it (two from the single bond and two lone pairs). However, we still have four valence electrons left. To make the outer atoms stable, we can distribute these four electrons as lone pairs on the central oxygen atom.
Ozone Lewis Structure How to Draw the Lewis Structure for Ozone YouTube
Ozone (O3) is an allotrope of oxygen and contains three oxygen atoms. In the lewis structure of ozone, there are one double bond and one single bond. Also, there are charges in two oxygen atoms in O 3 lewis structure. Lewis structure of O 3 can be drawn by starting from valence electrons of oxygen atoms in several steps.

What Is the Steric Number of O3 CarinahasCooley
The Lewis Structure of Ozone has:* three oxygen atoms in a row. It is not a ring, although that might be tempting.* two resonance structures* a lone pair on.

Kevin draws a lewis structure for the molecule of ozone, o3. later on
This chemistry video tutorial explains how to draw the lewis structure of O3. It also discusses the molecular geometry, bond angle, hybridization, and formal charges of the ozone molecule..

Lewis Structure
Ozone O3 Lewis Dot Structure - YouTube A video explanation of how to draw the Lewis Dot Structure for Ozone, along with information about the compound including Formal Charges, Polarity,.
O3 Resonance Structures (Ozone) YouTube
O3 Lewis Structure In this post, we will be drawing the Lewis structure of ozone, O 3. The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level.

O3 Lewis Structure (Ozone) Chemistry, Ozone, Lewis
Lewis Dot Structure of O3 (Ozone) kentchemistry.com 24.7K subscribers 131K views 11 years ago I quickly take you through how to draw the Lewis Structure of O3 (Ozone). I also go over the.
O3 Lewis Structure, Polarity, Hybridization, Shape and Much More
Lewis Dot Structure for O3 (Ozone) Watch on 6 Steps to Draw the Lewis Structure of O3 Step #1: Calculate the total number of valence electrons Here, the given molecule is O3 (ozone). In order to draw the lewis structure of O3, first of all you have to find the total number of valence electrons present in the O3 molecule.
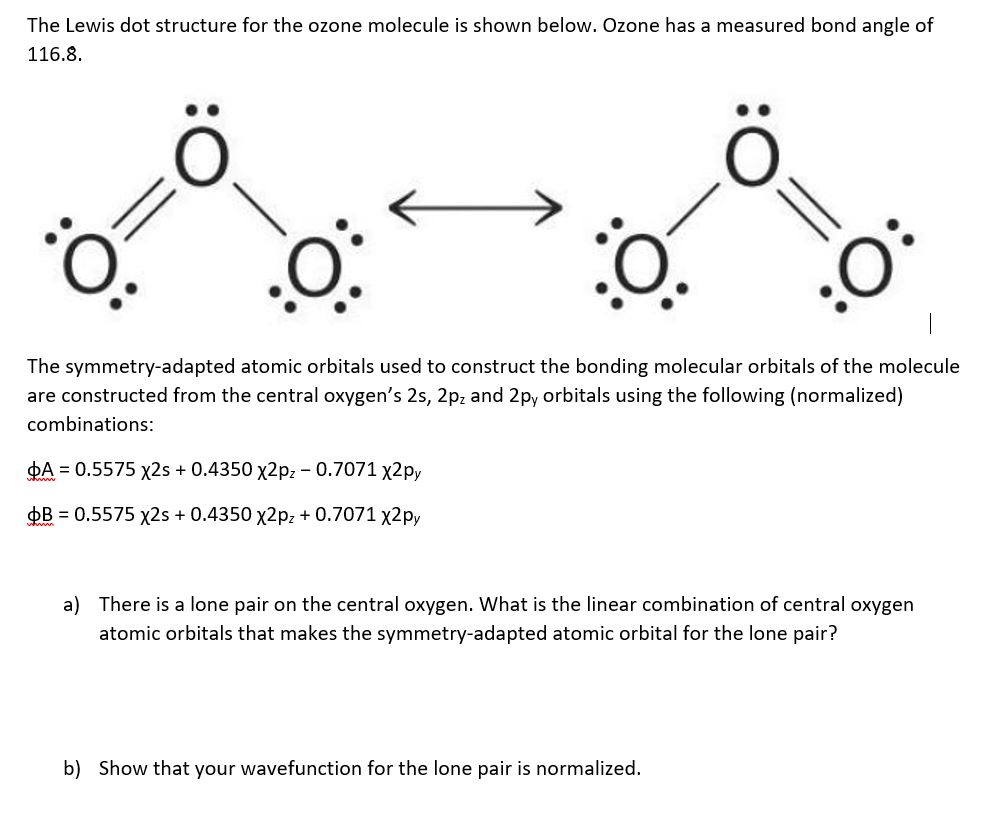
The Lewis dot structure for the ozone molecule is
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.
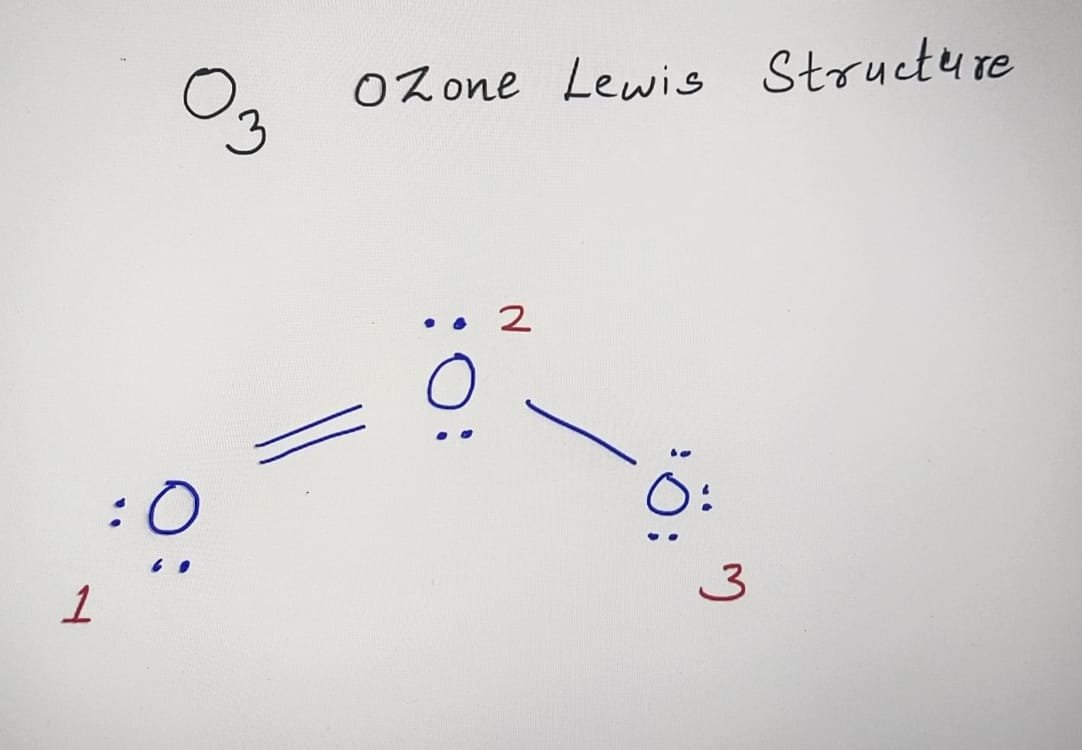
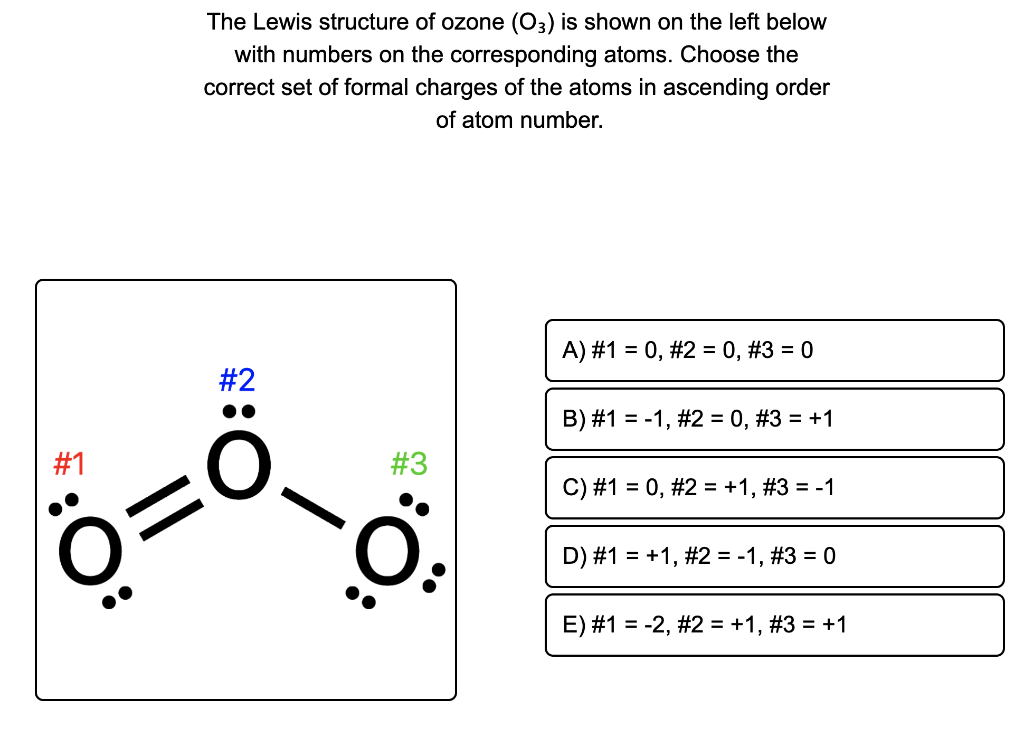
O3 Lewis Structure Formal Charge Basics of Chemistry
Sometimes one Lewis Structure is not Enough . Some molecules or ions cannot be adequately described by a single Lewis structure. For example, drawing one Lewis structure for ozone (O 3) gives us a misleading picture of the actual bonding in the molecule.If we draw a Lewis structure for O 3 (ozone), we get this:. This structure predicts that the two bonds are different lengths and strengths.
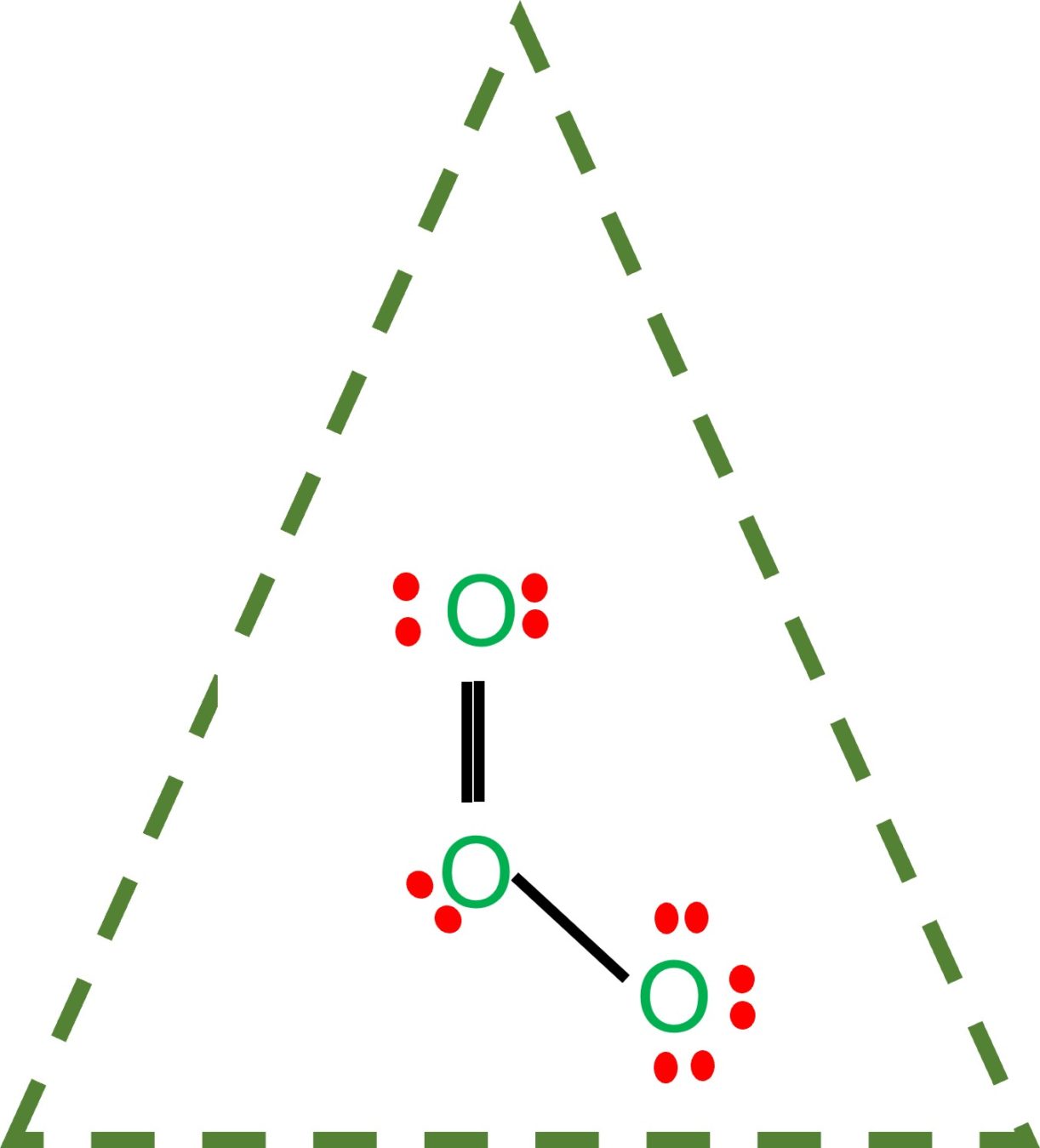
O3 Lewis Structure Step By Step Drawing What's Insight
Lewis dot symbols of the first two periods are given here to illustrate this point. In fact, the entire group (column) of elements have the same Lewis dot symbols, because they have the same number of valence electrons.. Draw Lewis dot structure for \(\ce{O3}\) and \(\ce{NO2-}\). Contributors and Attributions. Chung (Peter) Chieh (Professor.