Iso 13485 Internal Audit Checklist
+13485+Checklist.png?format=1500w)
ISO 13485 Gap Assessment Checklist — Medical Device Regulatory Guide
Ultimate Internal Audit Checklist: FDA QSR & ISO 13485 Audit Checklist See the Demo Ultimate Internal Audit Checklist: FDA QSR & ISO 13485 Audit Checklist Written by: Etienne Nichols October 25, 2023 It was nothing more than a checklist that saved $175 million and 1,500 lives.

Free ISO 13485 Audit Checklists SafetyCulture
Download now How do you create a checklist to check conformance? An internal audit is there to witness the outcome of a process through a review of records or witnessing the actions of the employees, and then to compare this to the planned arrangements for the process to see if what is being done is what was planned.
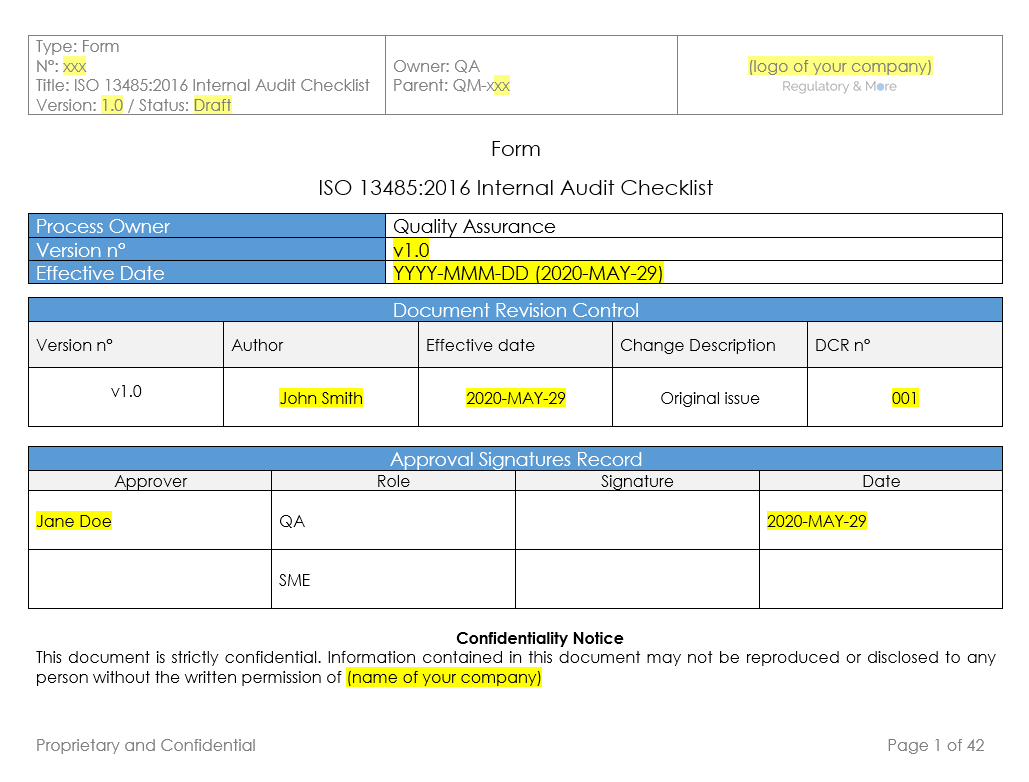
Checklist ISO 13485 2016 Internal Audit (v.1.0) Regulatory and More
The most effective method to comply with ISO 13485 is establishing a QMS and using an audit checklist to aid in certification. A digital QMS tracks, manages, and organizes all internal processes; and can be integrated with existing systems and solutions.

Iso 13485 internal audit checklist hitswes
ISO 13485 audit checklist . With that caveat out of the way, we recognize that often the best way to prepare for an ISO 13485 audit is a reassuring, tick-by-tick 'shopping list' approach as you assess your medical device QMS for weaknesses.

ISO 13485 2016 Internal Auditor Checklist TQS Inc.
An ISO 13485 audit checklist is utilized by quality managers to determine if the organization's QMS is aligned with the ISO 13485:2016 standard. It helps evaluate an organization's readiness for a third-party ISO 13485:2016 certification audit. With SafetyCulture (formerly iAuditor), quality managers can:
+ISO13485+Checklist+-+SCREENSHOT+WATERMARK.png)
ISO 13485 Gap Assessment Checklist — Medical Device Regulatory Guide
Procedures and Work Instructions: Forms and Checklists: Risk Management Templates: Document Control Templates: Training Records: Internal Audit Templates: Supplier Evaluation and Control Templates: Conclusion ISO 13485 is an international standard for quality management systems (QMS) specifically designed for the medical device industry.
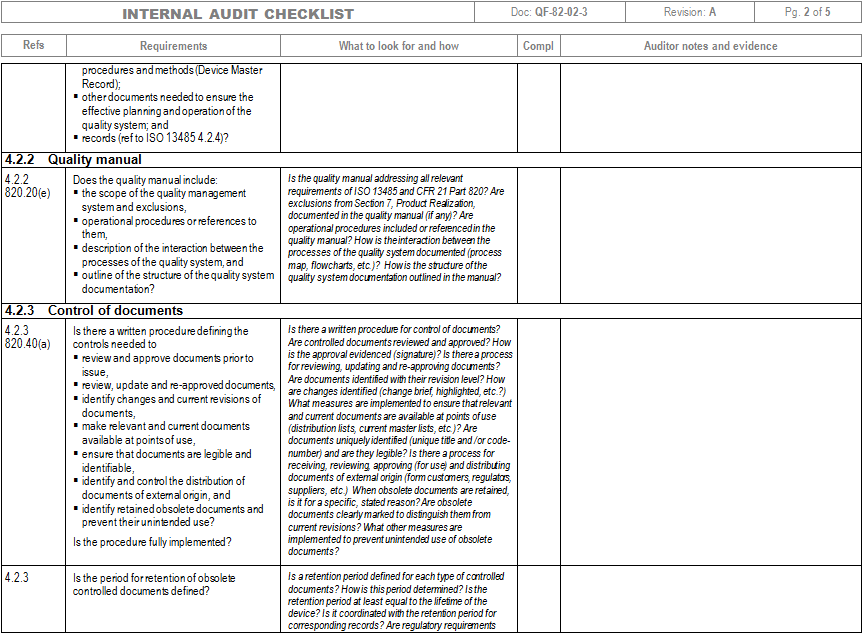
Iso 13485 Internal Audit Checklist
This checklist is based on the information provided in the 2016-03-01 release of the ISO 13485:2016 international standard. The checklist is best used by trained and practicing auditors to evaluate or assess Quality Management Systems requirements based on the standard.
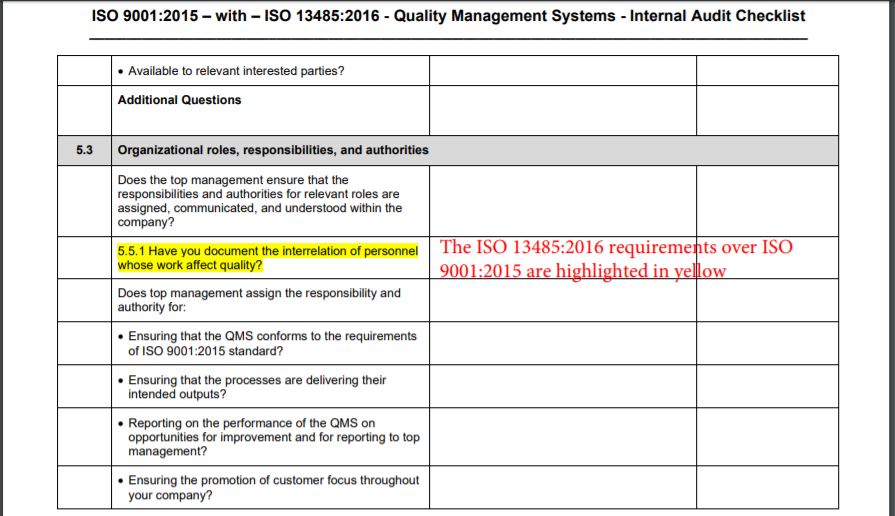
Iso 13485 Audit Checklist 2016 doctorazgard
Key steps in the internal audit process: Plan and announce the audit schedule. Plan the individual process audits. Conduct the audit. Report on the audit. Follow up on issues or improvements. What is an internal audit in ISO 13485? An internal audit is an important part of the Quality Management System (QMS) in ISO 13485.
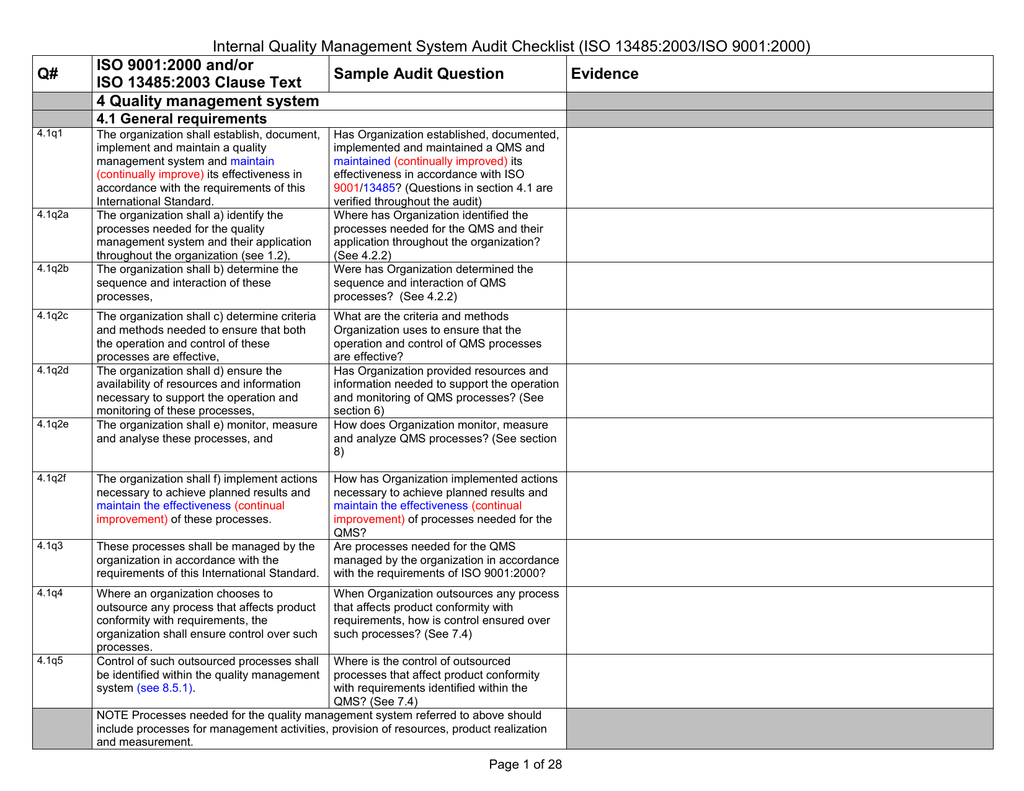
Internal Quality Management System Audit Checklist (ISO 134852003/ISO 90012000) Q
The audit checklist should include all relevant standard requirements of ISO 13485 and, in the best case, additionally the provisions of the Medical Devices Regulation 2017/745/EU (MDR) and/or the Medical Devices Directive (93/42/EEC). The template should be structured in such a way that all areas can be processed step by step without omitting.
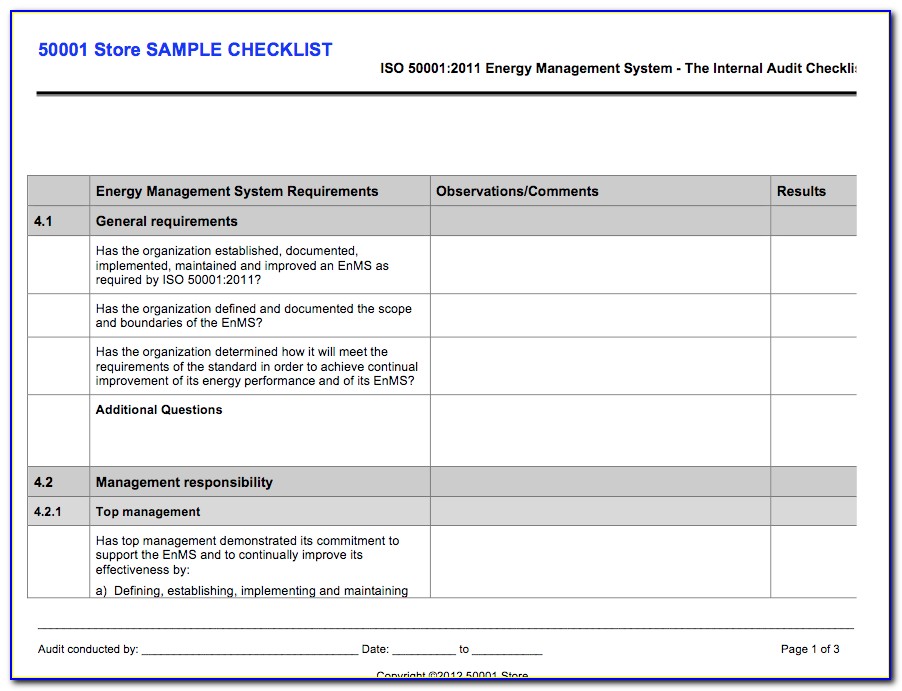
Iso 13485 audit checklist
ISO 13485 compliance checklist PDF Download guide ISO 13485 lays out the broad quality requirements for the modern medical device quality management system. Use this PDF checklist to ensure compliance with the particular and unique areas of the standard.
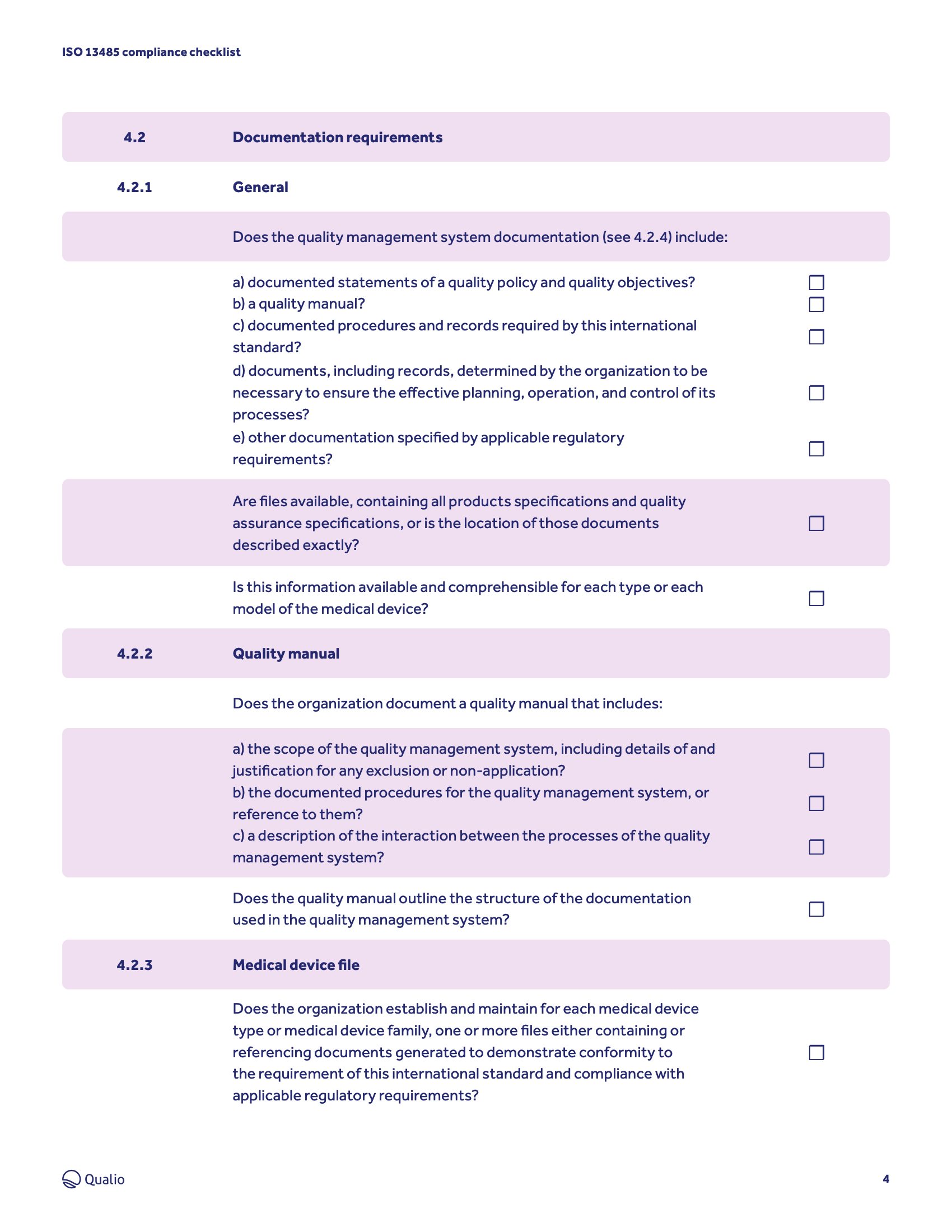
ISO 13485 toolkit
An ISO 13485:2016 standard checklist can help quality managers find gaps in the organization's current processes. This digital checklist is divided into 5 sections following ISO 13485:2016's key clauses. This converted digital checklist allows you to select "Done," "In Progress," "Not Started," and, for sections 6, 7, & 8, "Not Applicable."
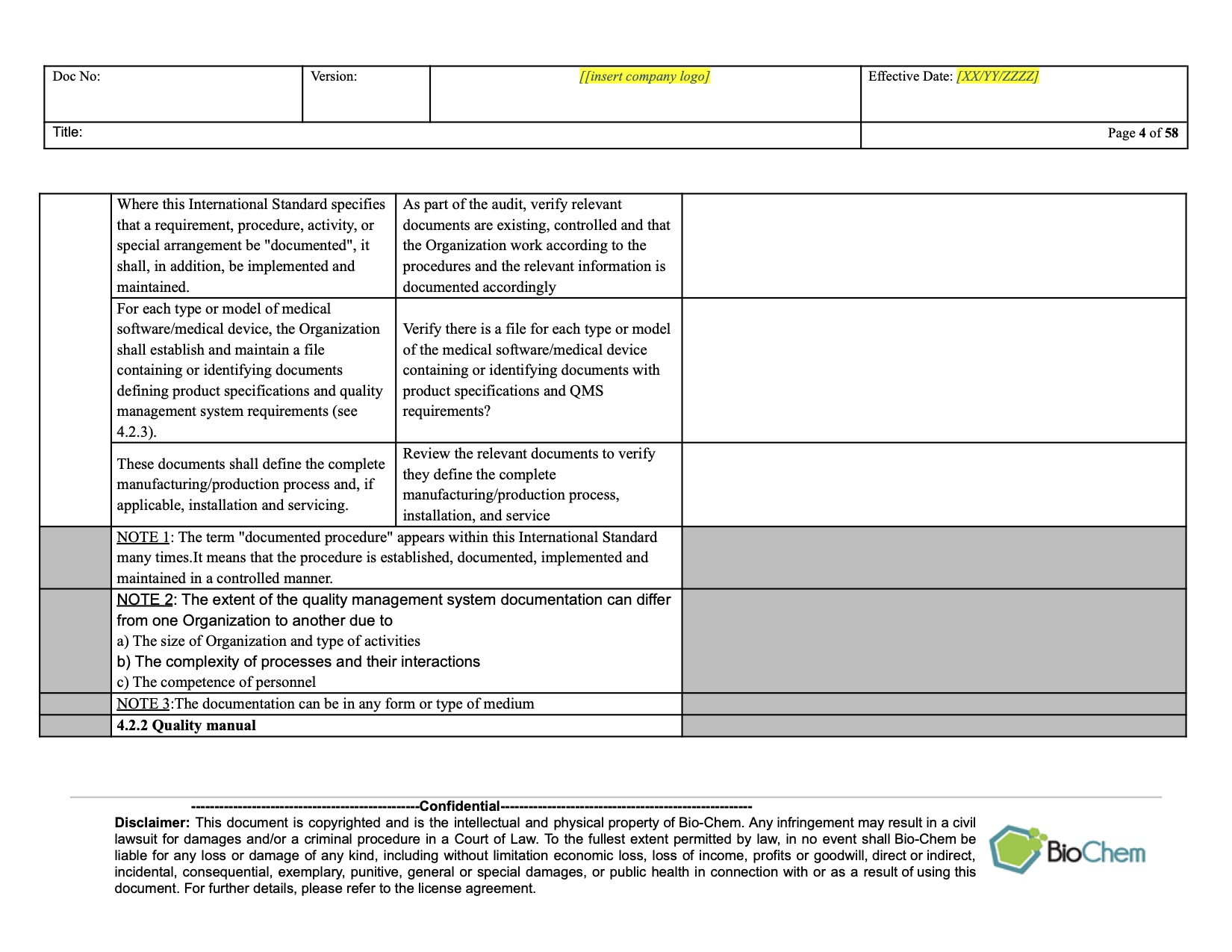
ISO 13485 Internal Audit Planning and Scheduling BioChem
For an efficient and straightforward internal audit process according to ISO 13485:2016, or in the framework of the preparation of a specific Internal or External audit, the ISO 13485 Audit Checklist is Ann essential tool. In the next section we will go through the characteristics of this compliance checklist.

Free ISO 13485 Audit Checklists SafetyCulture
This checklist is based on the information provided in the 2016-03-01 release of the ISO 13485:2016 international standard and on the code of federal regulations of 2016-05-26. The applicable parts of the regulation that result in additions or revisions for FDA are highlighted in yellow. The auditors are expected to keep in mind that the.

Kostenlose Iso 13485 Internen Audit Checkliste multichips
ISO 13485. This white paper is designed to help top management and employees involved in ISO 13485 implementation or transition, and to clear up any misunderstandings regarding the documents required by the standard. In this document, you will find an explanation of which documents are mandatory according to the ISO
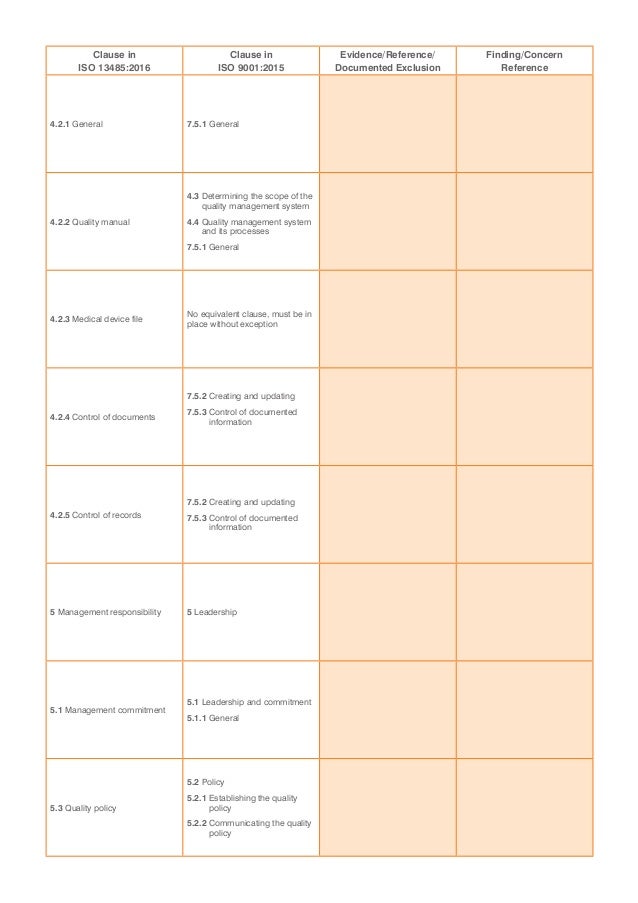
Iso 13485 checklist brospice
An ISO 13485 audit checklist is utilized by quality managers to determine if the organization's QMS is aligned with the ISO 13485:2016 standard. It helps evaluate an organization's readiness for a third-party ISO 13485:2016 certification audit.
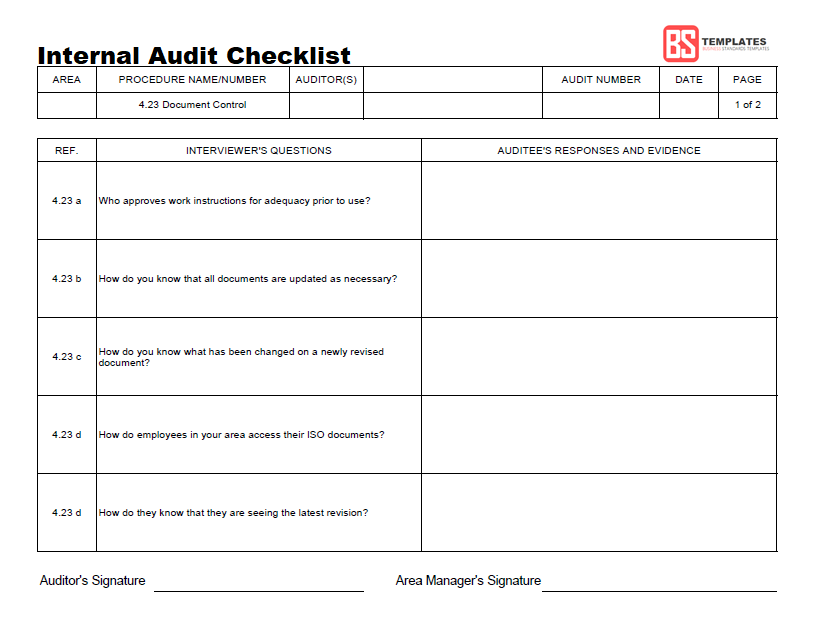
Internal Audit Checklist Iso 13485 locedfuse
ISO 13485 compliance checklist © Qualio — QMS for Life Sciences ISO 13485 lays out the broad quality requirements for the modern medical device quality management system. Use this checklist to ensure compliance with the particular and unique areas of the standard: Clauses 4 to 8. 4. Quality management system Item number Requirement Complete?