NO2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
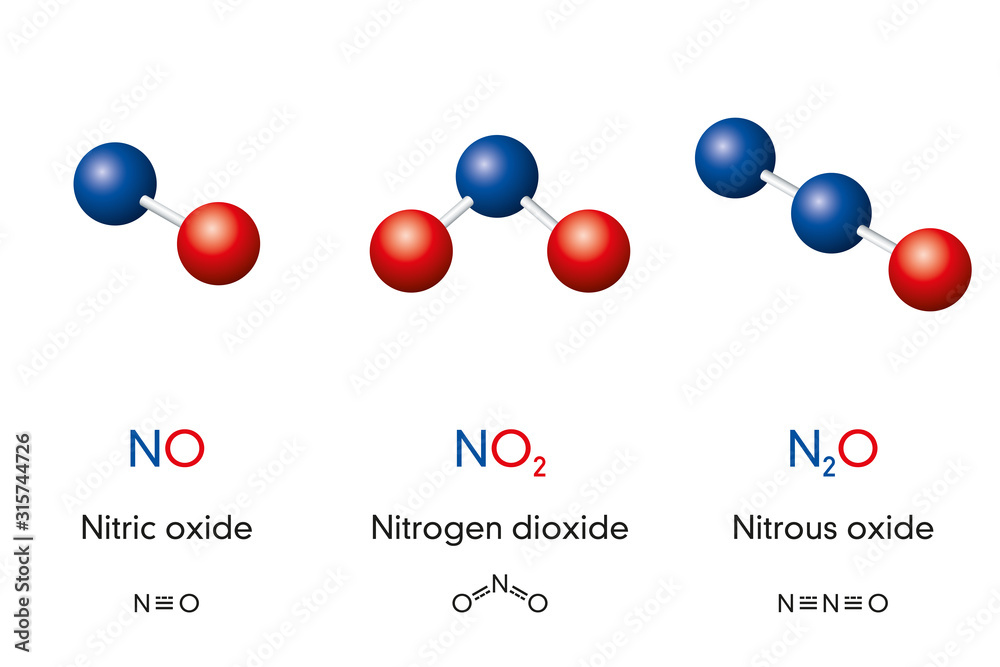
Fototapeta Nitric oxide NO, Nitrogen dioxide NO2 and Nitrous oxide N2O
The Lewis structure of a NO2- consists of a nitrogen (N) atom and two oxygen (O) atoms. The nitrogen (N) atom is present at the center of the molecular ion, while the two O-atoms occupy terminal positions. Meanwhile, there is a lone pair of electrons on the central N-atom.

Nitrogen dioxide, NO2, molecule model and chemical formula ⬇ Vector
Steps Sketch the structure Location of nitrogen and oxygen on the periodic table To sketch the NO 2 Lewis structure, the first step is to determine the total number of valence electrons in the molecule. This can be calculated by multiplying the valence electrons of each atom.

NO2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
Following steps are required to draw NO 2- lewis structure and they are explained in detail in this tutorial. Find total number of electrons of the valance shells of nitrogen and oxygen atoms and charge of the anion Total electrons pairs Center atom selection from nitrogen and oxygen atom Put lone pairs on atoms

No2 Lewis Structure
1.5K 128K views 3 years ago New AP & General Chemistry Video Playlist This chemistry video tutorial explains how to draw the lewis structure of NO2 also known as Nitrogen Dioxide..more.
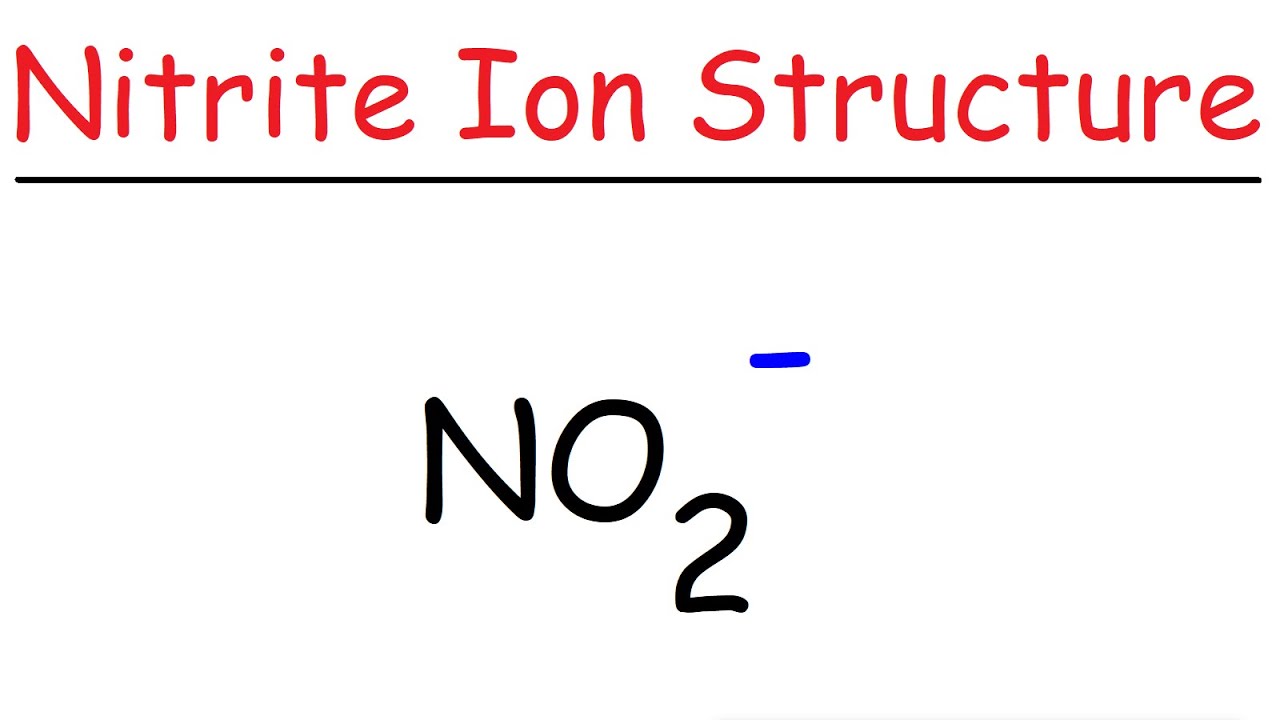
NO2 Lewis Structure Nitrite Ion YouTube
1.1K 208K views 6 years ago A step-by-step explanation of how to draw the NO+ Lewis Dot Structure (Nitronium ion). For the NO+ structure use the periodic table to find the total number of.
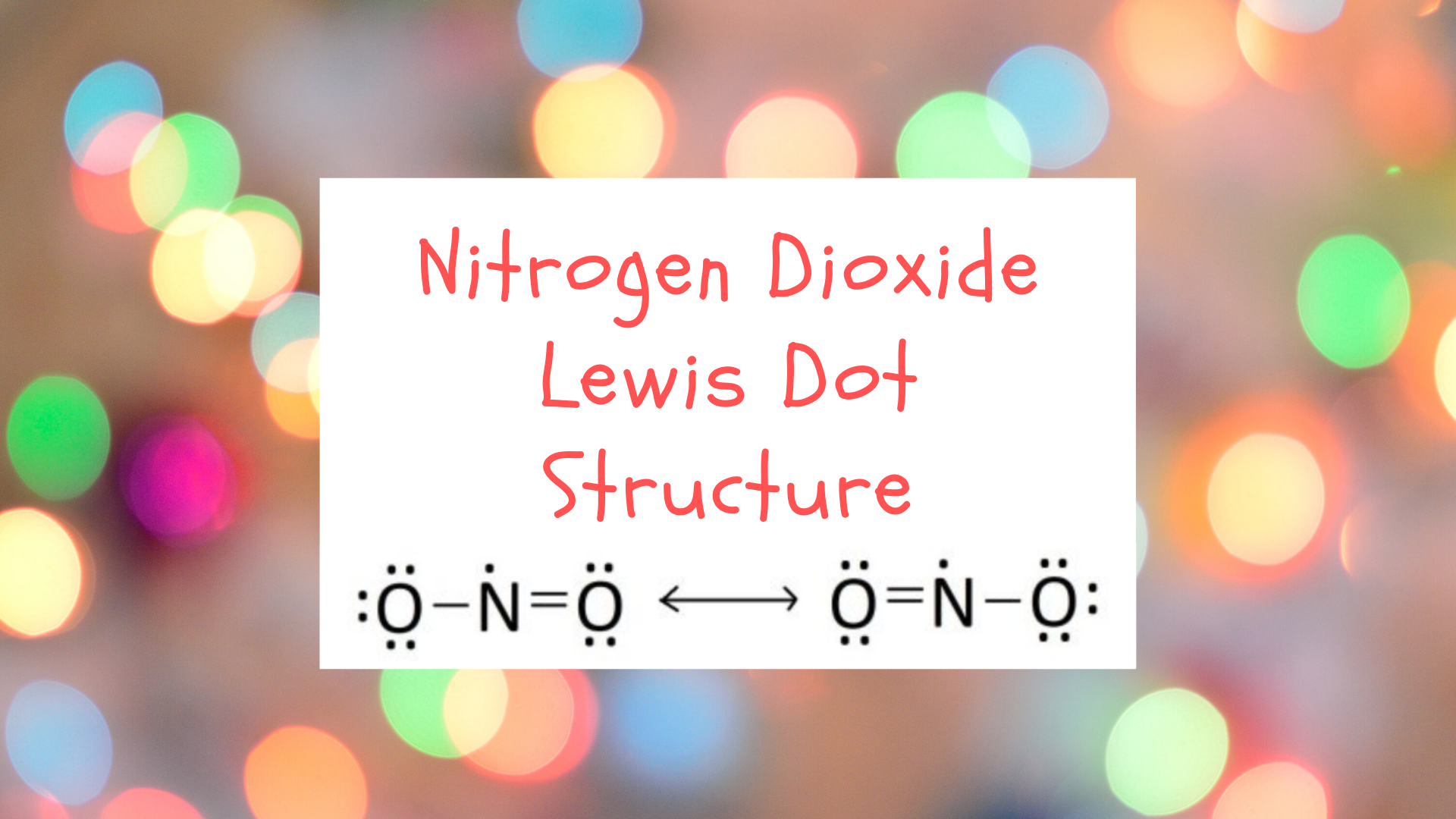
No2 Lewis Structure
Let us talk about drawing the Lewis Structure for Nitrogen Dioxide ( NO2 ). Lewis Structure of NO2 A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. Let us look at the periodic table. Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5.
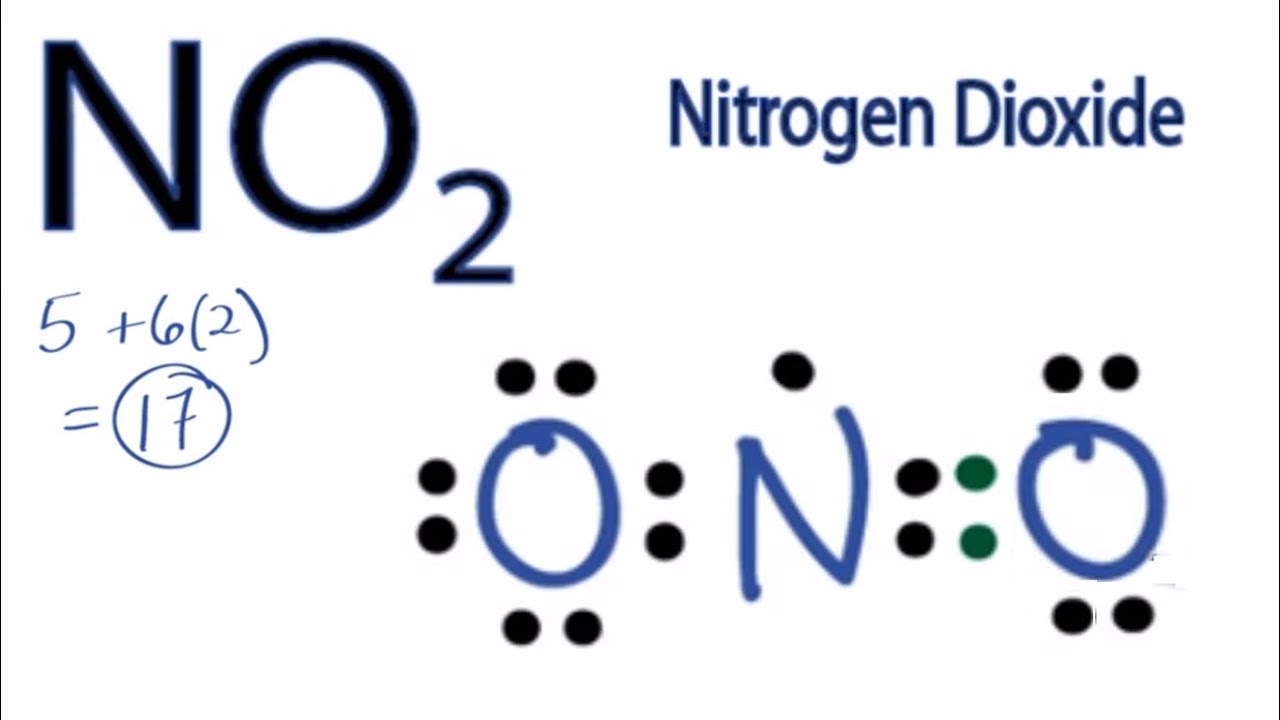
No2 lewis structure
NO 2 structure. The bond length between the nitrogen atom and the oxygen atom is 119.7 pm. That means both bonds exist between oxygen and nitrogen are same. Due to exist of unpaired electron, NO 2 is a free radical. There is a unpaired electron on nitrogen atom in NO2 lewis structure.
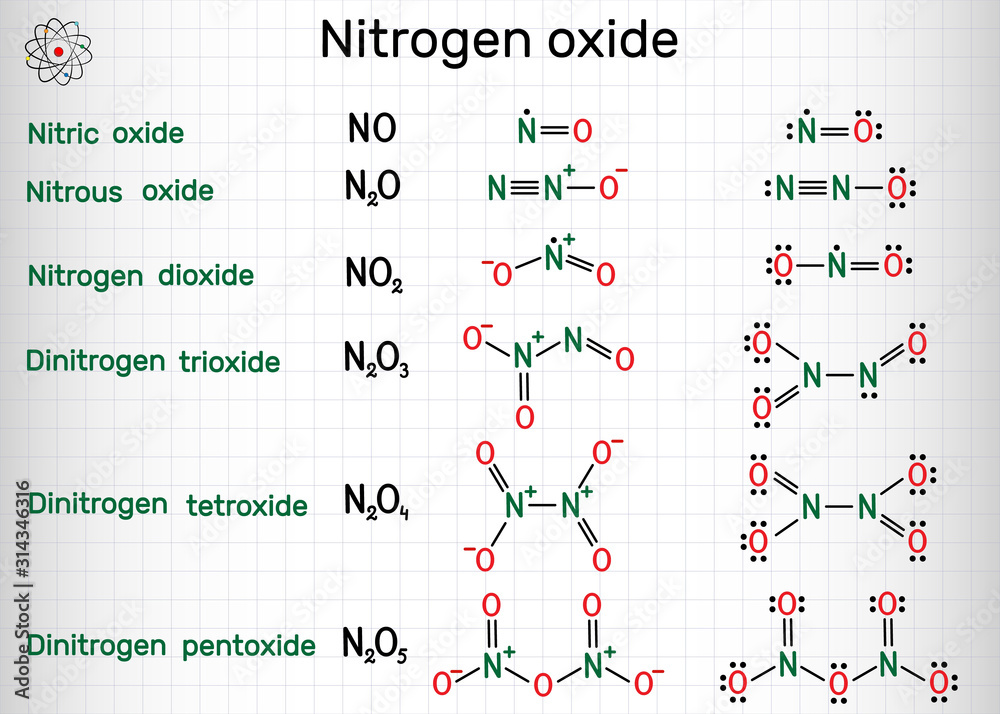
Chemical formulas of nitrogen oxide nitric oxide NO, nitrogen dioxide
This video explains how to draw the lewis structure of the nitronium ion NO2+.Chemistry - Basic Introduction: https://www.youtube.com/watc.

Diagrama de Lewis del dioxido de nitrógeno NO2 YouTube
NO 2 molecule: The Lewis structure of NO 2 molecule is shown below. Figure 1.2h NO2 molecule Lewis structure. For above molecules, they all contain unpaired (single) electrons. The neutral species that contain an unpaired electron is called radical (or free radical). When the carbon atom of a alkyl group has an unpaired electron, the species is.
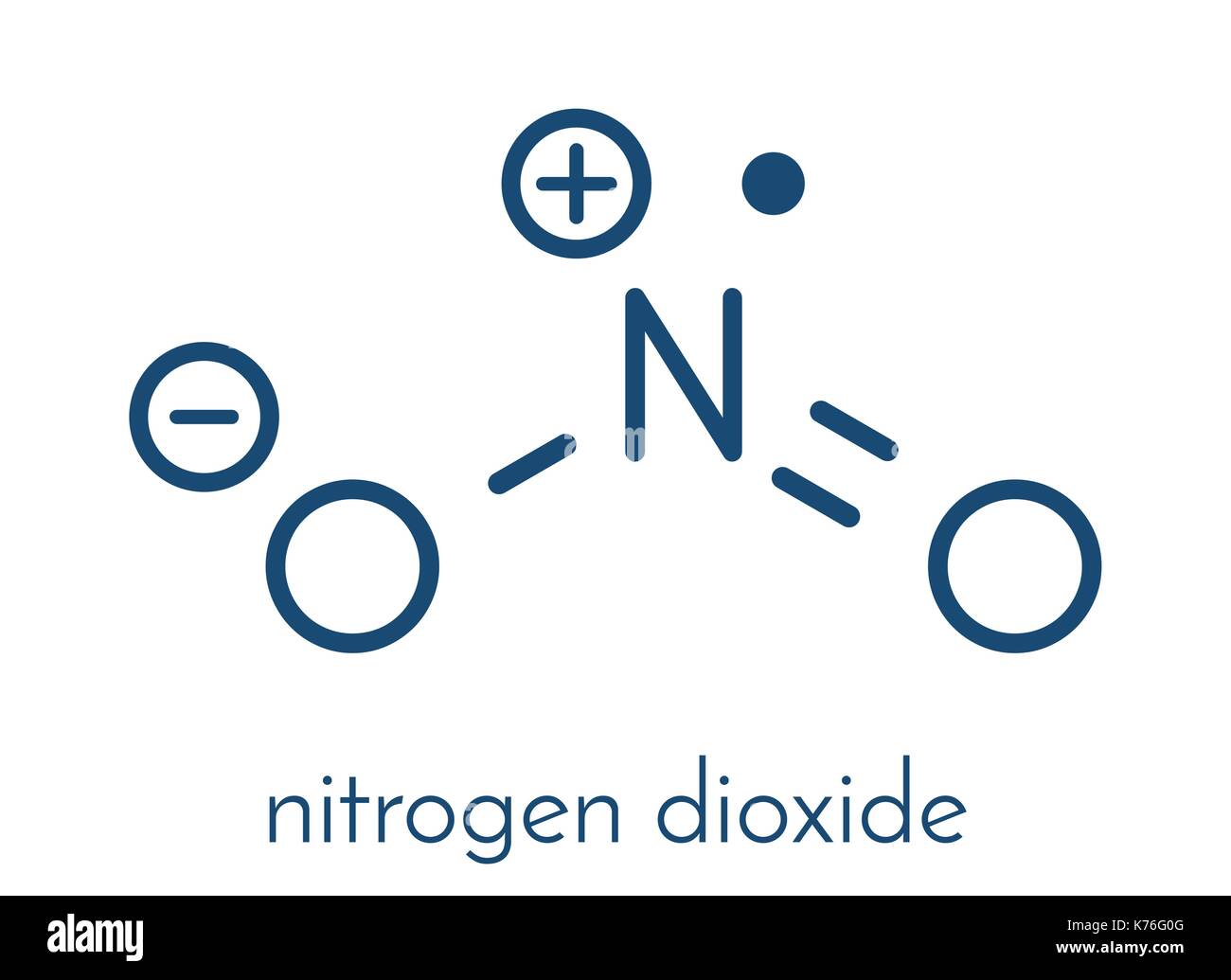
Chemical structure nitrogen dioxide no2 Stock Vector Images Alamy
NO2- lewis structure has a Nitrogen atom (N) at the center which is surrounded by two Oxygen atoms (O). There is 1 double bond and 1 single bond between the Nitrogen atom (N) and each Oxygen atom (O). There is a -1 formal charge on the single bonded Oxygen atom (O).

How to draw NO2+ Lewis Structure? Science Education and Tutorials
Key Takeaways The NO2 Lewis structure consists of a nitrogen atom bonded to two oxygen atoms. The nitrogen atom has a l one pair of electrons, while the oxygen atoms have three lone pairs each. The nitrogen-oxygen bonds are represented by single bonds, and the nitrogen-oxygen double bond is represented by a double bond.
How To Draw The Lewis Structure of NO2+ (Nitronium Ion) YouTube
The Lewis structure of NO2, which consists of one nitrogen atom bonded to two oxygen atoms with a double bond, influences its chemical properties. This structure implies that NO2 has a linear geometry and a formal charge of +1 on the nitrogen atom and -1 on each oxygen atom.

No2 nitrogen dioxide molecule Royalty Free Vector Image
Subscribed 2.4K 478K views 10 years ago A step-by-step explanation of how to draw the NO2 Lewis Structure (Nitrogen Dioxide). The NO2 Lewis structure has a total of 17 valence electrons..

Lewis Structure of NO2(1), the nitrite ion. YouTube
This chemistry video tutorial explains how to draw the lewis structure of NO2-, the Nitrite ion.Chemistry - Basic Introduction: https://ww.

NO2 Molecular Geometry / Shape and Bond Angles YouTube
Welcome to Warren Institute! In this article, we will dive into the fascinating world of Chemistry and explore the NO2- Lewis Structure. As aspiring
NO2+ Lewis Structure Nitronium Ion YouTube
A step-by-step explanation of how to draw the NO2 - Lewis Dot Structure (Nitrite ion). For the NO2 - structure use the periodic table to find the total number of valence electrons f.